2 Years For Vaccine Tech Transfers? COVID-19 Won't Wait

By Louis Garguilo, Chief Editor, Outsourced Pharma

A new McKinsey & Company report, titled “Why tech transfer may be critical to beating COVID-19,” indicates the typical time to tech transfer a new vaccine is 27 to 29 months.
“Over 2 years for a tech transfer?” I thought incredulously.
Certainly our drug industry has become more efficient at establishing manufacturing operations. Vaccine developers appear to be proving that in the midst of this pandemic.
Turns out I may be correct regarding what we are currently witnessing, but a contingent of industry experts set me straight regarding my initial incredulity.
Two Years And Counting
We are witnessing a remarkably fast-moving effort by the global biopharma industry – from Boston biotechs like Moderna to the Big Pharma vaccine operations of Pfizer – to develop a vaccine against the Wuhan coronavirus.
At the same time, we should recognize total efforts for vaccine research, development, approval, manufacture, and distribution – involving elongated clinical trials and regulatory approvals – can and often do traverse decades.
Yet even within this more typical timeframe, does it really require over two years for the “typical” tech transfer of a vaccine?
Here’s how McKinsey charts it:
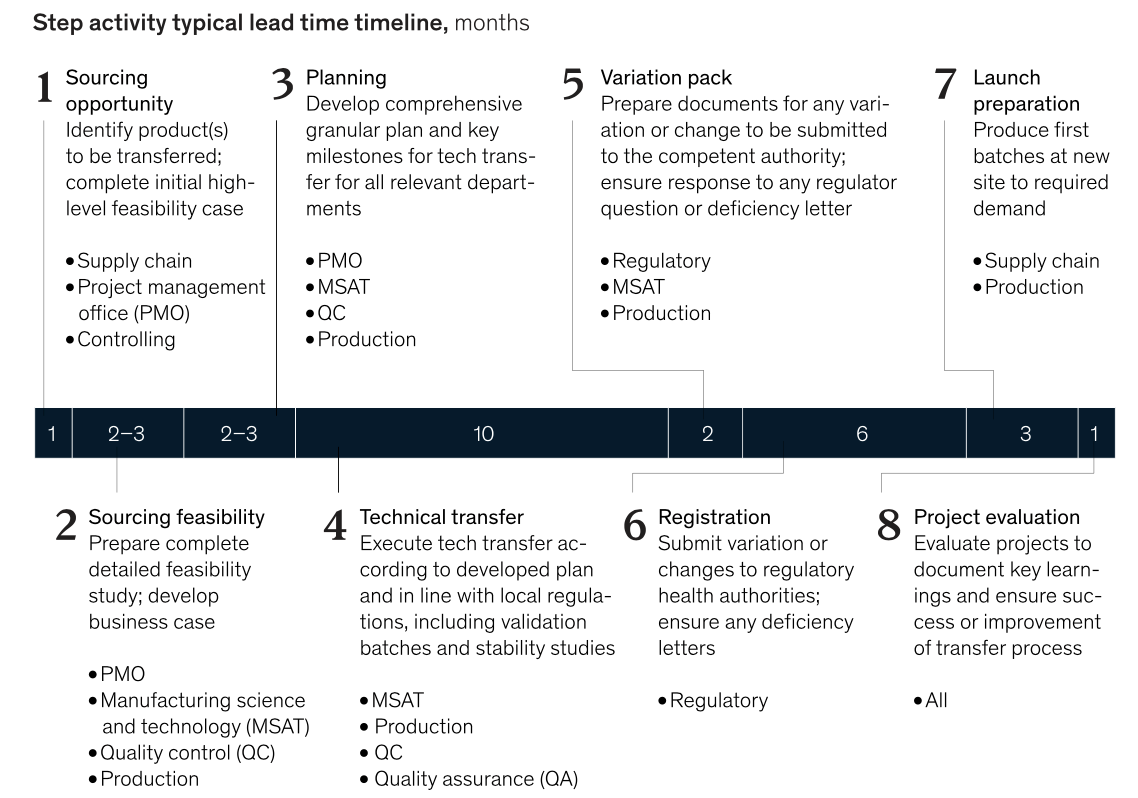
I reached out to professionals in my network for some independent confirmation of this timeline.
They all say the McKinsey authors are spot on.
Ernst Soethout, Managing Director, Virtuvax, with whom I wrote an editorial on vaccine development in 2017, immediately replied from the Netherlands.
“The topic raised by the authors is indeed a very relevant one, and deserves necessary attention. Tech transfer (TT) as a potential cause for delay of introduction of a COVID-19 vaccine has lacked necessary attention so far,” he wrote.
“It is a topic that should be further addressed, otherwise the whole process – start of vaccine production, market access, and eventual marketing – may all get delayed.
“That,” he added, “would be very difficult to accept by the public.”
David A. Dodd, Chairman, President & CEO, GeoVax, and an Outsourced Pharma Board member, sees it this way.
“According to this McKinsey report, which is based on a survey of vaccine manufacturers, the tech transfers “typically” take between 18 to 30 months.
“Obviously, it depends on the specific vaccine, and the complexity of manufacturing each, but theirs is a pretty good benchmark to use.”
Michael Watson, former President, ID Therapeutic Area and SVP Partnerships at Valera LLC, a Moderna Therapeutics’ venture focused on vaccines and infectious diseases, was the most circumspect in his initial thoughts.
“Tech transfer is highly dependent on what technology you are transferring, who/where you are transferring it to, and what resources both partners have available for the transfer.
“This,” he says, “is especially true for established vaccine platforms where the product is the process.”
Liliana Chocarro answered from Canada. She’s worked as senior staff and consultant for international agencies in the field of global health, specializing in regulatory evaluation of vaccines, and is currently President, LC Plus Consulting Inc.
“Yes, it may take 2 years or more, depending on the company chosen to produce, and the regulatory pathway used for assessment and approval,” she said.
Less Time For TT
“And yes, it is possible to shorten this now” continues Chocarro.
“Selecting experienced partners; agreements between academia and industry; support from organizations like WHO (World Health Organization), GAVI [The Vaccine Alliance] and CEPI [The Coalition for Epidemic Preparedness Innovations]; and discussions with regulatory bodies, all count to shorten the timelines.”
Watson adds another potential accelerant: “vaccine platforms for which the end products can be more easily characterized in their own right.”
“Specifically those platforms that use disposable technology, for which most recently built biological facilities can be relatively easily converted,” he adds.
“Platforms, such as mRNA [the technology employed by Moderna/Valera], will increasingly share components between vaccines, and for which standardized master files can be used to accelerate the process.
“Therefore,” Watson concludes, “the potential to go faster is there for the right vaccines and partners. In fact, two years itself is a significant improvement compared to a decade ago.”
To prove that final point, he recalls a presentation he gave at a WHO meeting in 2010, “when a 5-to-10 year window was the norm for a full vaccine tech transfer for large-scale manufacture.”
Therefore, we learn, a two-year tech transfer is itself targeting a timeframe already greatly reduced from where it was a decade ago.
And today, the world expects even better.
The McKinsey report offers its own analysis of how we can get to better, including this overview:
Best in class transfer Implications

Regarding these recommendations, Ernst comments:
“The authors provide some very sensible options to reduce tech-transfer time. The suggested solutions at this stage are still at high level, and therefore merit further practical arrangements.
“Possibly the ‘hackathon’ as suggested in the report would be an option to get quickly on track with such arrangements.”
Hackathon?
According to the report:
“One solution may be to create a team of ten to 20 professionals and organize a hackathon based on existing process maps to determine best practice for tech transfer, to create the knowledge-management infrastructure, and to apply digital and advanced analytics tools to transfers. In this way, we can respond quickly to any technical challenge and, in doing so, speed up transfers, enable faster supply of critical medicines, and help save lives and livelihoods.”
This sounds a lot like a tech-borrowed solution to find the “bugs” in the system, and implement the fixes to cure them.
Let’s hope this pandemic does become an accelerant for even wider industry coordination and cooperation.
Reality Check
Because the drug industry has nicely advanced in the art and science of development and manufacture, we tend to overlook the individual efforts and global coordination needed to safely and efficiently transfer projects to external production sites, forming a formidable global supply chain.
Coordinated outsourcing still requires our full attention.
Dodd of GeoVax concludes:
“Tech transfer is of course conducted as a routine activity within the vaccine business’ processes. My guess is with a highly focused effort and well-defined plan – following an at-risk tech-transfer effort – the timing can be reduced, and “under a year” is feasible, especially with an allocation of tremendous resources behind such an effort.”