"We're Not A Company Without Continuous Manufacturing"

By Louis Garguilo, Chief Editor, Outsourced Pharma
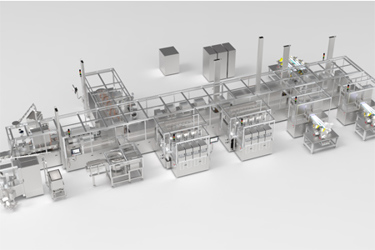
Lyndra Therapeutics is designing in-house a specialized, continuous and automated manufacturing line to manufacture its new long-acting oral-drug products.
Someday, it hopes to export that line to partners around the world.
The development and manufacturing strategy was driven in large part by CEO Patricia Hurter – who took over from co-founder Amy Schulman in 2019 – after her experience establishing the first continuous processing line at Vertex Pharmaceuticals.

Predominantly, that propulsion consists of the questioning and understanding of the pitfalls around a “typical pharma build.”
Those pitfalls exist in the transfer of learning, materials and equipment, from small to mid-size to larger batches, and to different professionals often at different locations, as well as in the time and money spent on repeated development and qualifying at each stage.

In fact, it was at a service provider where he first heard of Lyndra.
Not A Company If We Can’t Make It
Knox was working at a CDMO when he met the founders and first employees of Lyndra. They’d come looking for support in developing and building their early-stage product line.
The CDMO didn't have the capabilities. “It required specific equipment and skills. It would've cost them more to come through us than try to do the early work themselves,” recalls Knox.
Nonetheless, intrigued by the program (described in detail in part one), Knox stayed in touch with Lyndra for about a year and a half. “They kept asking me to join,” he says, “but I said, ‘you're not ready for me.’”
Finally, in 2016, he agreed to meet with cofounder and CEO at that time, Amy Schulman.
“Her comment back to me was, ‘Well, Ray, we aren’t a company unless we can make our product. Come on board, show that we can make it, and make it at volume.’”
“Having seen many other small companies, I knew the CEO was often the make-or-break person,” says Knox. “I realized she was going to follow through.” He took up the challenge.
Then in 2019, Hurter succeeded Schulman as CEO. She was all in on a continuous platform.
“So was my team,” recalls Knox.
The Same Continuous Page
In some regards, Knox says, “continuous manufacturing is about how long we keep the same piece of equipment turned on.”
“The first time you run a bunch of excipients and polymers and blend it all up, it may not be the way you're going to run it permanently.”
But once you reach “a state of satisfaction,” he says, “why wouldn't you just repeat doing that, versus the constant need to do all that process work, and come up with capital expenditure at each stage?
Increased output, then, no longer requires more work and capital to ensure it's the same quality product throughout.
Therefore, Knox says, “We turn around that basic scientific knowledge – instead of the scale-up cycle, continuous is a reasonable and economically sound approach to doing product development.”
And it can’t hurt when your new CEO declares, “We’re doing continuous manufacturing."
“That was great,” says Knox with a smile, “it met up with all of the expectations my team and I have always had.”
Knox mentions that many other industries already have (and have had for decades) automated lines that once switched on, attempt to run 365 days a year.
In parts of our industry, as well – syringe manufacturing for example – companies want lines running until no more product is needed or you shut down for a planned maintenance event.
“That's the mindset,” says Knox. “once you are able to monitor and manage your output to fully understand you are producing the same dose each and every time.”
Question Of Quality
The mindset, yes … but speaking of quality, it remains a topic of debate regarding continuous.
To which Knox replies: “The quality equation to me has always been a little bit funny.”
“The first time I got involved in a drug-device combination product, we brought people in from the pharma world. They were ‘educating’ us medical-device people.
But the quality perspective always got me: It didn't matter what the batch size was, they took 10 samples for quality analysis. ‘Why only 10?’ I asked. ‘If you make 5,000 units or 50,000 units, how do you show the uniformity?’
“I know there's QBD work in the background, but to me you should be continuously monitoring what you're producing to ensure consistency.”
“Today, our quality group feels that way,” continues Knox. “With the right tools, and if you've done the requisite upfront work, you can demonstrate by monitoring your processing parameters and certain aspects of the output in real-time.
“You can identify you've got product being made in the appropriate way.”
There are asterisks, though.
You must clearly establish the ability to recognize any “perturbation in the system” that then can be segregated for testing. Having an integrated quality plan along the continuous line is a key component.
Knox also spoke about analytical development – particularly challenging for Lyndra’s long-acting oral-dosage forms – and other elements of integration into continuous processing.
So there’s no intention here of lightly considering the efforts, equipment and knowledge workers it takes to introduce continuous manufacturing.
Particularly, perhaps, when utilizing the growing CM services being established at CDMOs.
But while over the years the editorials I’ve written on CM have often focused on the painfully slow adoption in our industry, Lyndra demonstrates we now have the future in the palm of our hands.
P.S. – Outsourced Pharma recently held a webinar on this very subject: Continuous Manufacturing: “Processing” A Project Near You
-----
Selected earlier editorials on continuous manufacturing:
FDA Leads, C-Suite Lags Continuous Manufacturing Acceptance
Continuous Manufacturing In The Mediterranean Sea
Keeping Up With Continuous Manufacturing
At Novartis, It’s The Quality In Continuous Manufacturing
Novartis And The Arrival Of The Continuous Manufacturing Facility
