Supply Chain Agility: Did You Have It Last Year? What About Now?

By Louis Garguilo, Chief Editor, Outsourced Pharma

Challenges seem more sanguine in hindsight.
There’s also this thought process: “We got through that, let’s move on; there’s catching up to do.”
And who wants to yet again revisit the challenges of 2020?
Perhaps we should – and focus that retrospection on this: supply chain agility.
Did you have it in 2020? Do you have it now? What is it, actually?
In an as-it’s-happening whitepaper based on a survey and interviews, IDC chronicled the thoughts of supply chain executives in the middle of last year’s COVID disruptions.
“Agility” rang out as a byword.
And evidence that in fact, you may have been considerably more worried than you recall.
Look And Act
The IDC whitepaper is based on an international survey of those with significant influence over their company's supply chain.
It was conducted in Q2 of 2020 and included 532 respondents: 49% were bio/pharma companies; 24% health systems/hospitals; 12% CDMOs; and the remainder retail pharmacies, wholesalers, and third-party-logistics companies.
Interestingly, for a whitepaper titled, “Supply Chain Agility In The Pharmaceutical Industry,” agility is considered an addend to a larger sum:
Visibility + Agility = Resiliency
These three components are described thus:
- The essence of resiliency is visibility into the supply chain and the ability to act upon what is seen – within appropriate time frames.
- Visibility is that capability to see what is happening throughout the supply chain at suppliers, inventory, business processes, and customers.
- Agility is the ability to respond within necessary time scales, for example adjusting/repurposing manufacturing capacity or inventory, or activating alternative suppliers and external partners.
- In other words: actionable visibility both upstream to suppliers and downstream to customers.
Unfortunately, pharmaceutical manufacturers tended to view this type of visibility as their most significant gap.
For Outsourced Pharma, readers this is particularly salient:
We had settled into the narrative CDMOs performed admirably for you despite the year in crisis, therefore demonstrating (although not precisely in these words) your supply chain agility throughout the pandemic.
So this is well worth another look.
Actionable Fear
We started here worried that 2020 concerns might be mitigated by the passing of time, as the pandemic played itself out. (Although not yet, and fingers firmly crossed.)
But throughout last year, biopharma executives were fully engaged and ready to enact supply chain adjustments to secure more agility as defined above.
Those priorities are listed in a specific question in the IDC report.
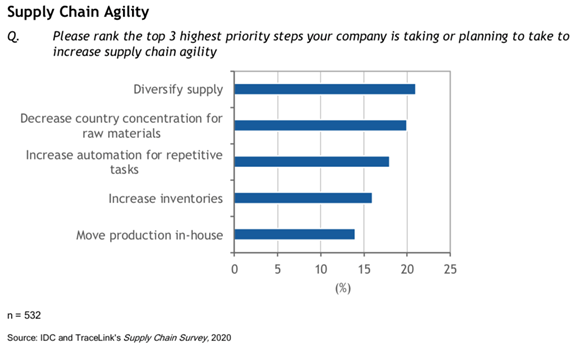
Hopes are many of these actions have been or are being taken up as you read this.
The IDC whitepaper points out inventory is perhaps the sole item that can be affected in the short term. The others are longer-term oriented.
Which should prompt readers to ask themselves:
Are agility goals for our supply chain – those we committed to during 2020 – in process and on track?
A Brief Worldview
Because the pandemic in fact did expose a lack of supply chain agility – and much was being said regarding globalization vs. localization – the whitepaper felt compelled to spend some time on this specifically.
However, much unlike this editor – a strong advocate for repatriating pharma supply-chains (to the U.S.) – the whitepaper is conversely concerned pharma (and other companies) avoid “knee-jerk reactions” resulting in poor choices about long-term, supply chain strategy.
This particular discussion is not to be pursued presently, but worth this brief note.
The report wraps up the subject thus:
“Striking the right balance between globalization and localization will present businesses with both opportunity and challenge.”
And we assume, the proper agility.
Other Considerations
Here are some overall observations the IDC whitepaper makes based on its survey and follow-up interviews, illustrating the depth of concern at the time.
The pharma industry was “significantly affected” by COVID-19.
Seventy percent of respondents at the time (mid-2020) felt their supply chain was “extremely vulnerable to the continuing pandemic.”
Supply-chain executive were experiencing spreading drug shortages, and “significant degradation of delivery performance from suppliers…”
And the whitepaper makes this submission:
“The pharmaceutical supply chain is marked by generally poor visibility, particularly from an end-to-end perspective, with efforts more aspirational than actionable or achieved.”
Seventy-eight percent of companies felt having [more] timely, finished-goods visibility would dramatically reduce drug shortages – a top challenge for half the survey respondents.
Overall, supply chain agility appeared quite limited in scope, with 43% of respondent companies confirming they lack “the necessary agility and redundancy to survive major business disruptions.”
It’s also worth noting respondents thought their “adoption of technology” was inconsistent, “with significant collaboration challenges upstream and downstream.”
Forty-five percent stated upstream issue-resolution exceeded 40 days, and 29% said downstream resolution-issues did as well.
The whitepaper concludes supply-chain transformation efforts “are mainly reactive, or focused [too narrowly] on a specific, functional-area performance, rather than on end-to-end supply-chain solutions.”
(My) Conclusion
It’s obvious increased supply chain agility will go a long way in assisting our drug development and manufacturing outsourcing industry overall, as well as individual organizations. In normal and abnormal conditions.
And regarding our coverage of the pandemic:
As we sit here today and look back, perhaps our Outsourced Pharma community was not overly serene throughout last year.
We registered what readers and our network of outsourcing professionals were telling us: times are very challenging, but we are making out relatively well.
Could the more dire-straights results from the IDC whitepaper, and our reporting, both be accurate?
I believe so.
While mounting challenges confronted readers, there was a calm determination, and an aggregate of agility within your drug development and manufacturing supply chains to at least initially overcome those challenges.
It was both those difficulties and that capable resolution we were registering throughout 2020.
And so the question remains: How supply chain agile are you feeling now?
