In Year 20, "Biopharmaceutical" Remains The Same

By Louis Garguilo, Chief Editor, Outsourced Pharma

Twenty consecutive years of most anything to do with our fast-paced industry is, on the face of it, a span to be applauded.
This year, BioPlan Associates released its 20th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production (A Study of Biotherapeutic Developers and Contract Manufacturing Organizations), to perhaps a little more fanfare than usual.
The good folks at BioPlan allow me to review the report and present some takeaways to Outsourced Pharma readers.
We’ll eventually get to an interesting chart related specifically to outsourcing drug development and manufacturing included in this 470+ page report, but let me start with nomenclature.
There are two points of naming differences between BioPlan and Outsourced Pharma. I think taking a moment to discuss them with you will be meaningful.
Bio + Pharma + Alpha
From the report (ellipses are mine):
What do we mean when we say Biopharmaceutical?
‘Biopharmaceutical’ refers to pharmaceuticals, generally therapeutics, manufactured using biotechnology (manufacture by living organisms), the related processing involved is “bioprocessing,’ and the related subset of the ‘pharmaceutical’ industry is the biopharmaceutical industry or sector …
This 20th annual version of this publication, as noted above, continues to use a consistent, bioprocessing professional-friendly definition for “biopharmaceutical” and “bioprocessing.” Over the years, the traditional meaning of the terms has grown and evolved to be more synonymous with the pharmaceutical industry as a whole … This distinction as the “biopharmaceutical” industry as a subset of “pharmaceutical” will be maintained within this report to provide a more traditional biopharmaceutical or bioprocessing industry sector friendly definition.
This and other BioPlan publications remain among the few industry sources retaining use of the classic biotech-based definition of “biopharmaceutical;’ and among the very few with fully consistent historical data sets.
In other words, the professionals at BioPlan have been using the term biopharmaceuticals for some 20 years, and they’ll be darned if they are going to change meanings now.
For the sake of “historical data sets” that makes a ton of sense. But I hope that doesn’t also mean that here at Outsourced Pharma, we don’t.
Consulting with readers and others in my organization, a few years ago I decided to use “biopharma” as the catchall for our entire drug/therapy industry – small and large molecules, cell and gene, RNA, or whatever else comes our way – when discussing the industry as a whole.
I admit to some inconsistency in so doing, but I increasingly use it, as in, “Our biopharma industry increasingly relies on all sorts of CDMOs and other service providers for development and manufacturing.”
It’s noteworthy that BioPlan eschews the word – although it slips in on occasion within the report.
Why did we make the switch to an expansive use of “biopharma”?
You could say the BioPlan report actually starts to make our case for us:
As the industry continues to grow and expand into new and innovative technologies, publications, and biopharmaceutical industry itself will evolve to become more broad facing. The industry in its current form is already expanding to encompass emerging technologies in medical instrumentation, medical devices, diagnostics, and artificial intelligence (AI) applications. This suggests that “biopharmaceutical” has already become something of an umbrella term referring to biologics-related high-tech health care segments.
Not to quiver (although that’s what I’m doing), the real distinction in my mind is that biopharmaceutical denotes a drug, therapy or other related health-care product, and biopharma designates our entire industry.
And I believe we benefit from bringing all our divergent business operations together as a single industry.
What was once the pharmaceutical industry is now the ever-expanding biopharma industry. At least some of us think so.
Oh, and by the way, where does “biotechnology” now fit in?
Under the heading Methodology, the report states:
Note, “biopharmaceutical” here refers to the classic biotechnology-grounded definition: involving manufacture of pharmaceuticals using biotechnology/bioprocessing. The term does not refer to the entire pharmaceutical industry or just those parts considered innovative, with “biopharmaceutical” now simply commonly substituted where “pharmaceutical” or “drug” were formerly used.
Perhaps in the end, you need to be a scientist to figure out how we describe our industry.
Service Providers
One more such point to raise.
This year, for the first time in the history of our annual CMO Leadership Awards, we changed the name of the awards. They are now the CDMO Leadership Awards. (Note the “D”.)
Why? The contract organizations themselves have been asking us to do this for some time. Here’s how I explained this in the CDMO Leadership issue’s introduction this May:
This one-letter change makes a significant difference. Today, from Big Pharma to the widening array of biotechs developing drugs and therapies, there’s a universal outreach to external partners for assistance with developing new programs. Development and manufacturing have collapsed into a set service offering, if you will, and providers must integrate to fully serve their customers. So, to both sides of the outsourcing equation, welcome to the first CDMO Leadership Awards.
Actually, In Outsourced Pharma editorial, we made the switch to the universal use of CDMO many moons ago.
On this, BioPlan agrees. Sort of. Throughout their important report, “CMO” and “CDMO” is interchanged. I reached out to the survey authors, and received this reply:
“In the past, contract manufacturing in bioprocessing was focused more on the ‘manufacturing’ part, so the CMO moniker was most relevant. But as new technologies emerged, and the demand for earlier stage co-development of biologics became more critical, the ‘development’ side of manufacturing became an increasingly vital part of services offered. There are few pure CMOs today that only manufacture, so most would bill themselves as CDMOs. But they are all still CMOs – that do development support services.”
More Than Words
With that ... Let's now we get to the chart I mentioned above.
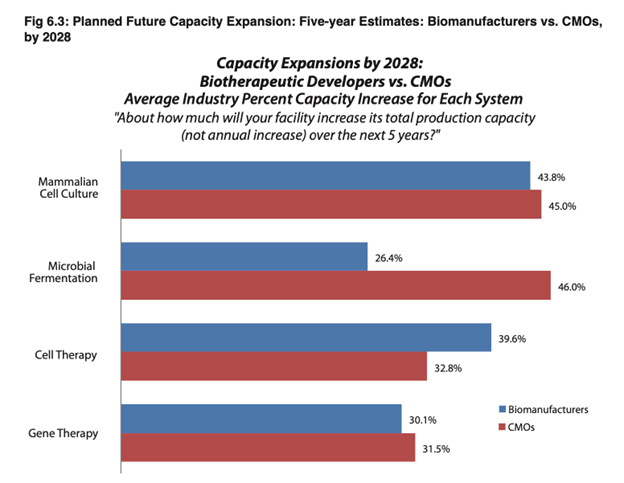
Describing the chart, BioPLan comments:
“Overall, again, this year CMO respondents indicate they expect to construct more capacity than Biomanufacturers in nearly all categories, except for ‘Cell Therapy.’”
Therefore, no matter what words we use to describe our evolving outsourcing industry, we’ll be using them a lot for the foreseeable future.
Now … what’s a better word for “outsourcing”?
