What Proposed Plasmid DNA Standards Say For GMP/Non-GMP Production
By Antony Hitchcock, principal and owner, AGH Bioconsulting
The production of many advanced therapies, including viral vectors and mRNA products, requires genetic code for the design and assembly of viral vectors and for the therapeutic genes they carry. In most cases, this code is in the form of plasmid DNA produced in bacterial systems. It, therefore, follows that plasmid DNA has been identified as a critical starting material in the production of these products.
The standards used to produce plasmid DNA have evolved over the last 20 years, as these products have moved from very small, academic-led studies to the current state where they show up in product pipelines and an increasing number of licensed products. Initially, there were minimal guidelines on plasmid DNA and variable standards of material were used, from research grade to GMP.
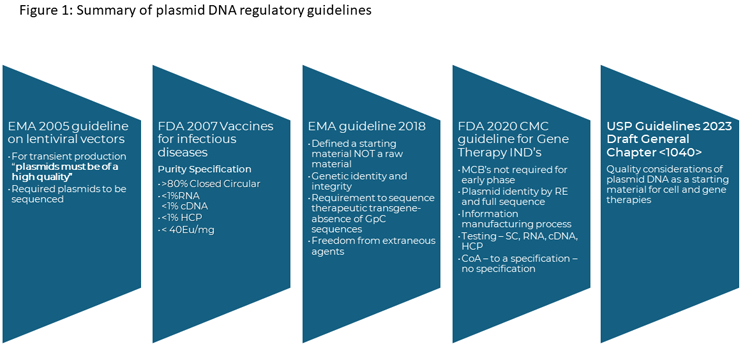
In 2005, the concept of high-quality plasmid was introduced by the EMA relating to the production of lentiviral vectors, which allowed vector producers to use non-GMP grade plasmids for vector production. However, the EMA provided limited guidance for what high-quality referred to, resulting in a wide range of interpretations.
Then, in 2021, the EMA released its Questions and answers on the principles of GMP for the manufacturing of starting materials of biological origin used to transfer genetic material for the manufacturing of ATMPs laying out expectations for the quality systems to be applied for plasmid DNA and identifying where different grades of plasmid can be used in clinical and commercial applications.
In November 2023, the United States Pharmacopeia issued draft General Chapter <1040> Quality Considerations for Plasmid DNA as a Starting Material for Cell and Gene Therapies. The draft updates previous guidelines released on the production of plasmid DNA both as a therapeutic product and as a critical starting material. These are summarized in Figure 1.
To better understand these draft guidelines, I posed several questions to USP's Rebecca Potts, Ph.D., who is the lead expert on the panel that developed the general chapter. I was particularly interested in how standards vary for GMP- and non-GMP-grade plasmids.
The document’s scope relates to the use of plasmids as a critical starting material for cell and gene therapy products, including the production of RNA vectors. It excludes vaccines and plasmid used as direct therapies, such as vaccines and for direct cell modification.
No Surprise Here — Industry Wants And Needs Manufacturing Standards
Fueling the general chapter’s development, USP stakeholders wanted more guidance on the manufacture and testing of plasmid DNA because existing standards are not detailed enough for cell and gene therapies and mRNA manufacturing, Potts told me. Why they’re not specific enough should not surprise anyone who’s been paying attention to advanced therapy development.
“With the increased number of new cell and gene therapies, the supply chain has been unable to adapt to the demand for high-quality reagents, including plasmid DNA — a critical starting material in many cell and gene therapies,” Potts said.
Beyond that, rare disease indications often receive accelerated review windows, which shorten the time typically devoted for process development, she added.
Interestingly, the draft general chapter excludes synthetically-produced plasmid DNA, which requires a plasmid template and often — although not exclusively — is produced in a bacterial system. In my experience, cell-free platforms offer flexibility, which may encourage some companies to opt for the fully synthetic approach, which could sharpen demand for more standards specific to synthetic plasmids in the future.
Facility Standards For Plasmid Manufacturing
Facility design plays a part in <1040>, and while the guidelines are clear for GMP facilities, there is less about non-GMP facilities.
Potts said the GMP facility should be designed to provide adequate:
- segregation,
- engineering controls,
- air and material flows,
- equipment layout,
- equipment selection,
- gowning and cleaning, and
- controls to prevent cross-contamination by foreign DNA.
“Where feasible, single-use equipment should be used to reduce the risk of cross-contamination,” she said.
Now, that’s for GMP manufacturing. A key difference between GMP and non-GMP facilities is the “degree to which procedures and risks are documented and controlled.” GMP plasmid DNA is more often produced with single-use technologies, while non-GMP plasmid is made in vessels that are cleaned and reused, she said.
In my experience, however, that’s not always the case. Many non-GMP suppliers have, in fact, switched to single-use platforms largely because customers are concerned about cross-contamination, and this is also reflected in expectations around facility design and operation.
In terms of phase-appropriate use, EMA guidelines give scope for non-GMP plasmid to be used for some commercial use (e.g., lentivirus used for cell modification); however, the USP general chapter, while referring to the EMA guidelines, does not seem to give that option.
Potts said that would be better addressed by regulatory authorities overseeing a user’s clinical development program. She noted that the chapter states that, “Plasmid DNA starting material used in Phase 3 clinical trials should be made in a full cGMP facility, and certain clinical trial host countries require cGMP. However, plasmids used in commercial manufacturing must follow cGMP.”
It is clear that many AAV vector producers have already decided that all late-phase material should be made with GMP-grade plasmid DNA, and these guidelines confirm this position, restricting the use of non-GMP plasmids to Phase 1/2 clinical studies. The situation is less clear for lentivirus producers who need greater volumes of plasmid for cell modification.
QMS And Vendor Qualification For GMP And Non-GMP Programs
Quality management systems are a critical component of GMP compliance. While industry has clear guidelines for GMP systems, instructions are less clear for non-GMP systems. However, customers increasingly hold producers to higher standards for non-GMP plasmids.
Plasmid supplier qualification should be part of the user’s quality risk management system, Potts said, adding that an end user who is using the plasmid to manufacture a CGT product is responsible for performing quality audits to ensure the supplier meets its quality system requirements.
When comparing GMP and non-GMP standards, not all processes are overseen by a QA group for non-GMP compliant processes, Potts said. Also, the quality control assays are established assays but not qualified or validated in non-GMP compliant plasmid production.
It is clearly incumbent on end users to perform audits on suppliers, and, one assumes, they should be prepared to justify quality standards used to their regulators, including around analytical systems. End users will need to have an in-depth understanding of the risks associated with plasmid production and the potential impacts on their products.
Staff Training
USP did not include training standards in <1040> and considered them out of scope for this chapter. Training requirements would be unique to each manufacturer’s QMS, Potts said, adding that staff should receive sufficient training.
This is clearly going to be an area where end users will need to make assessments during audits with regard to the levels and documentation of training that they require of potential plasmid suppliers.
Stability Studies
Finally, the general chapter proposes standards for stability studies, which are a key requirement for GMP materials but are not frequently performed on high-quality plasmids because of high costs and material demands. “The chapter does not specify stability testing be performed on either GMP-compliant or non-GMP-compliant plasmids, but the chapter recommends users perform stability testing to assign an appropriate use period,” Potts said.
Overall, when combined with the EMA guidelines, it is apparent that officials in the EU and U.S. are sufficiently concerned about the standards and plasmid supply in clinical and commercial applications.
Potts makes an interesting point in that the EMA and FDA fast-tracked many products under accelerated review, which limited development times.
Shorter development times meant smaller windows for changes within manufacturing processes.
The change in usage from a non-GMP- to a GMP-grade plasmid supply inevitably affects clinical development programs. At one level, it affects cost and time with regard to plasmid production, which may take up to 12 months. It also may involve a change in suppliers, manufacturing processes, and, potentially, the generation of new cell banks, which, in turn, may impact the plasmid purity.
Many companies that use non-GMP plasmids are currently seeking to mitigate risks by using GMP cell banks and processes that are scaled-down versions of GMP processes, with the same process steps and raw materials.
For plasmid producers, the effects of the new general chapter and EMA guidelines raise the bar for these services, including facilities required, quality systems, and operational costs. Producers may face viability questions when calculating the extra setup or upgrade costs against the market size and likely profit margins on batch sizes that are often less than 1g.
To make this a viable offering and meet required standards, suppliers likely will streamline their offering with existing GMP services to minimize duplication of staff and activities. In terms of production facilities, the production scales for non-GMP are often smaller than for GMP services, so it is likely that separate production facilities will continue to be required. In terms of using the same facility to produce both grades of material, Potts says suppliers that produce both grades of plasmid in the same facility would need to demonstrate that the materials and manufacturing systems are appropriately segregated.
The question going forward for end users will be about how and when to use high-quality material. For some applications there is a logical answer, such as, for example, in the production of lentivirus, where the material needs are low and multiple steps from the patient, whereas for AAV vectors where the material demands are high and closer to the patient, the decision may favor the use of GMP materials throughout. It may also drive vector producers to switch to stable cell lines and eliminate plasmids from their production processes.
In conclusion, draft General Chapter <1040>'s quality standards for plasmid DNA, along with the EMA guidelines, provide greater clarity for end users. However, we will have to see how suppliers respond from technical and commercial perspectives and how higher standards affect price levels and availability.
About The Author:
 Tony Hitchcock is the principal and owner of AGH Bioconsulting. He has spent his decades-long career in the in the biotechnology field, with over 30 years in the production of complex biologics for clinical trials in the European Union and U.S. He has worked in areas of process development and manufacturing with experience in engineering and process systems. He has worked on the development of more than 30 products for clinical trials, including plasmid DNA, viral and bacteriophage products, and recombinant proteins from microbial, mammalian, and insect cell sources. Contact him at tony@aghbioconsulting.com.
Tony Hitchcock is the principal and owner of AGH Bioconsulting. He has spent his decades-long career in the in the biotechnology field, with over 30 years in the production of complex biologics for clinical trials in the European Union and U.S. He has worked in areas of process development and manufacturing with experience in engineering and process systems. He has worked on the development of more than 30 products for clinical trials, including plasmid DNA, viral and bacteriophage products, and recombinant proteins from microbial, mammalian, and insect cell sources. Contact him at tony@aghbioconsulting.com.