Upping Flow Of Advanced Technologies Into Commercial Biomanufacturing
By Jeffrey C. Baker, Ph.D.

A good metaphor is a terrible thing to waste.
We live in a world of rapid change and complexity that demands bullet points and the reduction of multivariate situations to binary choices. So, a good metaphor that can help us get our heads around these matters is a pretty useful thing. This is particularly true when we consider the deployment of new technologies in the development and commercialization of biopharmaceuticals and, perhaps even more so, for modernization and continuous improvement of existing commercial facilities. I think I’ve stumbled upon a pretty good metaphor to help us think about this topic, so stick with me for a few paragraphs while we get there.
The development of flexible, robust, and economically efficient manufacturing facilities for biologics, especially those that bridge the molecular life sciences and the digital age, is a topic increasingly front-of-mind among innovators, investors, legislators, and even a lay public that is increasingly aware of global supply chain vulnerabilities. The innovation machine for target identification, analytics, clinical data analysis, and materials fabrication is alive and well, yet when we look at the use of modern, advanced manufacturing technologies in actual honest-to-goodness commercial manufacturing, we see technologies, metrics, and philosophies not too different from those in place 10, 20, or even 30 years ago. Everyone seems to want advanced manufacturing technologies in biotech, and numerous groups are pitching new approaches, yet here we are.
Why Not Biopharma AMT? ‘The Regulatory Burden!’
Why are we not seeing advanced manufacturing technology in commercial biopharma manufacturing? There are lots of ready answers, but a frequently-heard one is, “It’s the global regulatory burden!” It’s true that regulators do require documentation of what the manufacturing process looks like when it’s running correctly, usually involving numbers followed by units (we call that data), and do indeed require that assurance be provided that process changes to stable commercial assets are not clinically relevant. This does not seem too high a burden to me and, in fact, regulatory agencies, especially FDA, have active programs to incentivize and pull forward technologies that increase robustness and data-driven, patient-centered manufacturing. I won’t footnote extensively here (I need to get to my metaphor), but you can use your pocket computer that also makes phone calls to quickly search advance technology guidances and committees.
Some will say advanced manufacturing technologies put rapid review and approval at risk, but as I review inspectional issues and complete response letters (CRLs) of biopharm manufacturers, rarely do any involve advanced manufacturing technologies. In fact, one can only imagine how modern practices might have prevented CRLs that have been issued related to environmental monitoring and aseptic practices or data integrity. Global regulatory divergence and modal regulatory divergence are issues for our industry that unnecessarily increase complexity and overhead, but the idea that new technology in a manufacturing site might generate questions from a reviewer that delay or compromise launch is pretty thin soup.
Why Not Biopharma AMT? ‘It Costs Too Much!’
We might also hear, “It costs too much, and we must keep drug costs down!” I won’t even start with the economics of high-value low-volume manufacturing and risk management through automation and technology or run down the rabbit hole of manufacturing costs being fully uncoupled from drug pricing. I will, however, observe that if you are minimizing cost rather than optimizing value you will never deploy new manufacturing technologies. The cost of recapitalization and risk-adjusted modifiers to NPV, ROI, EVA, and the other spreadsheet-friendly discrete measures of cost will always be negative in a world of project-to-project and quarter-to-quarter budgeting despite the value of the same actions across portfolios and the derisking of failure modes over time.
Increasingly, we view manufacturing less as a value center and more as unfortunately necessary overhead to be done on the cheap. That is a choice of business model that merits informed analysis, but it is unclear why the biopharm sector is driven by cost rather than value in manufacturing, yet it deploys with passion inverse rhetoric for product pricing.
Perhaps we should take some degree of satisfaction that biopharma manufacturing is viewed as sufficiently commoditized that people think cost of labor per hour is actually relevant to value realization.
Why Not Biopharma AMT? ‘It Puts The Timeline At Risk!’
A NIIMBL Active Listening study2 described a third reason for a lack of AMT in biopharmaceutical manufacturing: “We don’t have time! Any risk to the timeline, especially for manufacturing reasons, is unacceptable!” This resonates across the industry, but it begs what the right-hand side of the timeline is for your endeavor. In the early days of biopharmaceutical development, the right-hand side of the acetate (remember acetates?) was often the third year of robust, reliable manufacturing or the second-generation process where we applied the learning gained and paid for in start-up and launch. End-in-mind thinkers modeled and worked backward to consider manufacturing options such as site sourcing and technologies and life cycle management, which might even be included in submissions and pre-approval supplements. Increasingly today, the right-hand side of the timeline is the next milestone, the next renewal or infusion of funds, the next submission or amendment, or the next Thursday staff meeting.
Aggressive deployment of sound Lean principles in the name of efficiency has also contributed to trimming of timelines (how many days after the last dose in a clinical trial will we submit and launch?) that rarely have coefficients of variance built into the many dependencies. Companies today are highly incented to divest of the burdens of physical and human capital,
which provide resilience to these variances. All change puts the timeline, that timeline developed by the folks that don’t actually have to manufacture anything, at risk, unless, of course, that change decreases variation, likelihood of failure, resilient recovery in the instances of failure, or assurance of flexible manufacturing capabilities to meet the needs of emerging products or unanticipated healthcare needs, considerations that also rarely appear on the right-hand side of the 21st century timeline.
What to do? How can we accelerate deployment of advanced manufacturing technologies for biopharmaceuticals in such an environment? How can we incent and activate value-based rather than cost-based manufacturing analyses? How can we do end-in-mind development in a short-planning-horizon, milestone-based culture? I’ve got an idea. Let’s be sneaky and co-opt established milestone, cost-based tools for end-in-mind outcomes.
Technology Maturity Models From NASA To NIIMBL
Technology Readiness Levels (TRLs) were originally developed by NASA in the 1970s to aid in Technology Readiness Assessments (TRAs) for new, rapidly developed and deployed, high-pain-of-failure technologies. Current standards for TRLs are used by the U.S. Department of Defense, the European Space Agency, and the EU Horizon 2020 Program and were adopted in ISO 16290:2013. Despite this broad use and utility, application of TRLs has been criticized when applied to sectors other than space, frequently because reliability of associated supply chains and economic factors were not included in performance assessments. Manufacturing Readiness Levels (MRLs) were developed by a joint DOD/industry working group under the sponsorship of the Joint Defense Manufacturing Technology Panel (JDMTP).
MRLs were adopted by the Department of Defense in 2005 as a common metric and vocabulary for assessing and discussing manufacturing maturity, risk, and readiness. Whereas TRLs assess the maturity of a technology, MRLs assess maturity, supply risk, robustness, and suitability for commercial manufacturing. Although well documented and firmly embedded in U.S. government contracting and granting practices, MRLs were not always useful for biopharmaceutical manufacturing because of the global regulatory environment, use in clinical practice, and process control requirements extending beyond final product specifications.
In 2022, NIIMBL proposed a set of Biomanufacturing Readiness Levels (BRLs).1 BRLs were intended to offer a shared vocabulary for the development and derisking of technologies for biomanufacturing and to aid technology developers in understanding expectations for manufacturing-ready technologies. Subsequently, significant effort has been applied to surveys, questionnaires, assessments, and categorization of technologies using BRLs. Less has been directed toward root-cause analysis of why advanced manufacturing technologies remain the passions of universities, process development teams, and expert conferences, rather than the tools of on-the-floor making of medicine.
Kant Said, ‘Metaphors Comprise The Conceptual Spectacles Through Which We View The World’
Rather than extending the checklists and definition of gates, let me offer a metaphor for thinking about technology maturity and value realization in commercial manufacturing. Simply put, it’s all about flow. Figure 1 shows my simplification of the complex BRL assessments available elsewhere and Figure 2 describes this metaphorical flow.
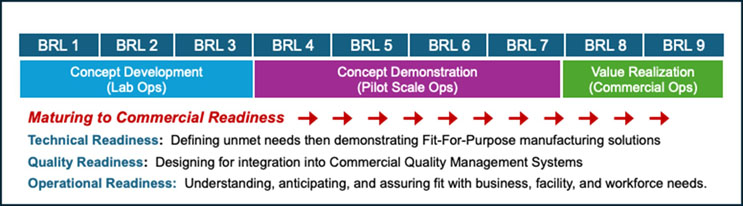
Figure 1. Summary of the NIIMBL BRL Model describing stages of technology maturity and identifying milestones in progression toward readiness for commercial operations. This model is described in much more detail by Kedia et al.1 and on the NIIMBL website.
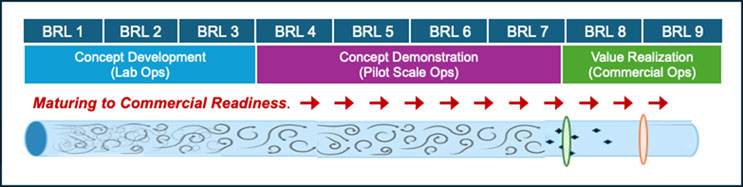
Figure 2. In the real world, technology does not mature in the linear, step-by-step manner described in Figure 1. We may instead describe maturation as turbulent flow through many pathways and stages with some technologies advancing further faster than others and others simply not advancing. One may envision selective membranes prior to BRL8 (manufacturing startup operations) and BRL9 (established commercial operations) that pass or exclude technologies based on factors discussed. In this model movement of technologies into manufacturing is not a matter of upstream concentrations or kinetics but of the characteristics of technologies and the imagined selective membranes. In this metaphor, acceleration of deployment of advanced manufacturing technologies into commercial biopharmaceutical operations is best facilitated by anticipating these characteristics and, perhaps, to extend the metaphor, develop facilitated active transport or chaperoning of technologies into commercial settings. In this churning, turbulent flow, it is important that exclusion criteria are based on data-driven constraints or clinical relevance rather than resistance to change or cognitive bias.
Consider the life cycle of a manufacturing technology as populations of nascent technologies flowing from left to right, maturing as they flow until they reside in commercial manufacturing. It would be great if it were linear flow and all of the technologies hatched made it to maturity, but we know it’s not that way.
Iterative development causes technologies to reside in different stages for different periods of time, and some advance to the next stages of maturity and some don’t. In this model, success in reaching commercial manufacturing is getting to BRL 8 and 9. In my imagination, I add a set of selective membranes to the NIIMBL model, one before commercial manufacturing and another before modernization of existing processes and plants, that filter technologies moving toward commercial deployment. Fueled by grants and publications and startup VC monies, it appears some think surely learning and momentum will carry X% of new technologies into manufacturing or at least to the next milestone almost through concentration-driven osmosis, but the membranes don’t allow that. Technologies have properties (real and perceived) that are passed or excluded by a whole different set of screens and constraints (real and perceived), adjudicated by a whole different set of decision makers than the technology champions, decision makers who are tasked with managing complex commercial situations and who can be extraordinarily risk-adverse toward manufacturing.
I don’t think the key to deploying advanced technologies in biopharmaceutical manufacturing is making more and more technology candidates or saying, “You know, if I just derisk this enough the industry will see how great it is.” I don’t think the answer is fully sequestering technology and process development from manufacturing organizationally, vast networks of university collaborations or more incubators, or making lists and taxonomies of the attributes of proposals and ideas. I think moving forward is about understanding the properties of the metaphorical membranes.
For my engineering friends it’s about linear flow, driving forces and systems-based thinking. For my biochemistry friends, we have a selective membrane and we need to enable active transport. For my business colleagues, every day in development we spend money, and on the other side of the membrane, we sell stuff and make money, so where does one invest? For my regulatory colleagues, what are the characteristics of technologies (dare I say “platforms?”) that do and don’t penetrate these membranes for reasons of compliance, and are they clinically relevant?
Managing Flow For The Plant And The Patient
When I think about the maturing of advanced manufacturing technologies toward commercial use, I no longer think about driving flow by increasing upstream pressure, that is to say, funding more and more early-stage tech development or shots on goal, but rather I think about accelerating flow by anticipating and relieving restrictions such as compatibility with production quality management systems, rapid switchover time with little or no product wasted in qualification, appropriate and well-understood cleaning and maintenance and, most of all, contribution to robust, reliable, resilient manufacturing that is not just an end in and of itself but a launch vehicle for the advanced manufacturing technology that produces for the patient.
The most direct path to an outlet, the one with the least churn, is linear flow and the most efficient way to deploy new tech is to prioritize focus on those opportunities that address the unmet needs of the plant and the patient and lay aside those that get trapped in eddies of incremental optimization.
After we have streamlined flow, we need to focus on attributes of technologies and hurdles to deployment. Of all the characteristics of technologies, membranes, and transporters, which ones are patient-centered and clinically relevant, and how can we focus on them? Which ones are cost-centered versus value-centered? Which are a function of data-based probabilities and which are the shadows of very human, risk-averse cognitive biases?
Diffusion is kinetically unsatisfying. We need to develop aids and programs that generate active transport across these membranes, and it begins with characterizing and understanding them in a data-driven way.
Manufacturing technology is not an end in and of itself but rather a bridge between current situation and desired situation. Its value resides in its utility. Desired situation is robust and resilient commercial manufacturing of biopharmaceuticals that is flexible, reliable, and resilient. I suggest getting there is all about understanding flow.
References:
- Kedia et al., Biomanufacturing readiness levels [BRL]—A shared vocabulary for biopharmaceutical technology development and commercialization, Biotechnology and Bioengineering, 119, 3526– 3536. https://onlinelibrary.wiley.com/doi/10.1002/bit.28227“
- NIIMBL-facilitated Active Listening Meeting between industry and FDA identifies common challenges for adoption of new biopharmaceutical manufacturing technologies” Jennifer L. Mantle and Kelvin H. Lee PDA Journal of Pharmaceutical Science and Technology May 2020; DOI: https://doi.org/10.5731/pdajpst.2019.011049
About The Author:
 Jeffrey Baker, Ph.D., worked in the biopharmaceutical industry for over 20 years in process development and manufacturing and served for 10 years as the deputy director in the FDA’s Office of Biotechnology Products, CDER. He has retired from FDA but remains active in the biotech community working with NIIMBL and NASEM and several universities.
Jeffrey Baker, Ph.D., worked in the biopharmaceutical industry for over 20 years in process development and manufacturing and served for 10 years as the deputy director in the FDA’s Office of Biotechnology Products, CDER. He has retired from FDA but remains active in the biotech community working with NIIMBL and NASEM and several universities.
