3 Updated Fundamentals Of Outsourcing

By Louis Garguilo, Chief Editor, Outsourced Pharma

Going on two decades, I’ve been directly involved in or interviewing, analyzing, and always closely following the art and science of drug development and manufacturing outsourcing.
The focus has been on improving outsourcing outcomes. That often means pursing innovations, trends, new best practices for relationships, strategies, and operating models.
Today, though, let’s look both back and forward – at how the time-tested basics of outsourcing endure, but with some evolutionary points of emphasis.
Here, then, are three key factors for increasing your chances of personal, programmatic, and organizational success, as you pursue the activities known collectively as drug development and manufacturing outsourcing.
- Two-Way Communication
I don’t believe I’ve ever conducted an interview with a professional in our industry who when asked for their advice for outsourcing most effectively and efficiently, didn’t include communication as one of the top subjects, if not the very first.
In fact, it became such a pat reply, I used to ask interviewees for their list of best practices but prohibit them from saying “communication.”
Of course by the end of those interviews, indelibly we’d be talking about that subject. So these many years later, I start our list where it started when I began writing editorials and moderating conferences.
There is a constant amplification of the theme today. It may seem redundant (and editors dislike redundancy), but I’ve added “Two-Way” to this best practice.
Can there be communication that is only one way? you may ask.
Apparently, there are many attempts at it. With ever-more data and increasingly complicated development programs, sponsors need to share all they know with CDMOs. For their part, CDMOs need to be fully transparent as to what is transpiring at their facilities.
Objectives, missions, quality systems, operational management. These require a back-and-forth dialogue. Trying not to sound like a school teacher here, but listening to what the other side is telling you until your side understands it, is critical.
“The reaction is hard to control, and inputs and processing improvements should be looked at now,” says the CDMO.
“More money is needed, and we must push out timelines,” hears the sponsor.
The resolution? Talk about ideas for potential improvements, discuss the impacts of extended timelines, look into how much funding changes will require – and if they will in fact save both time and money now and in the future. Be considerate to the challenges on either side.
Odds are for as long as I write editorials, I’ll be writing about communication. It may change in form, e.g., communication delivery systems – video conferencing, virtual inspections and audits during COVID. But the fundamentals will remain.
Today, I’m told, communications begin internally, with clearly articulating objectives and goals, expectations and timelines, and budgets.
Finally, the most critical time for "communication best practices" is prior to when, unfortunately, something goes wrong. With openness established, you can achieve the fast-and-furious professional communication needed between sponsors and CDMOs when there is a sudden disturbance along the development and manufacturing continuum.
- Flexibility and Agility:
Unlike numero uno above, this second key outsourcing component has gradually shot up the list. In fact, it wasn’t on the top of many lists a decade ago.
What changed?
To answer in a newly coined word: leaniality. (Yes, I just coined it.)
You operate leaner than ever before, start-up and maintain a “virtual” approach, run development programs with what used to be considered skeleton staffs, and perform wholesale and end-to-end outsourcing.
Next, what you pursue has changed, from steadfast small-molecule chemistry and then biologics, to cell and gene therapies, RNA therapeutics, protein degraders, cyclopeptides, antibody-drug conjugates, to name some.
You target small patient populations and rare and ultra-rare, “orphan diseases,” and at accelerated paces.
Most important to you:
- Is the CDMO flexible enough to work within my parameters and serve my individual program needs?
- Is it “agile” in that it can swap in and out small and limited programs – and then reintroduce them when intermittent processes need to restart?
- Are contracts adaptable to start-up sponsors and organizations like ours, and are financial negotiations tractable?
- Have the CDMO’s skill sets evolved with the new technologies needed for cell- or gene-therapy pursuits, and is the workforce stable?
Happily today, we can answer these questions. Service providers appear, for the most part, to be listening, and evolving alongside sponsors.
Recent editorials have brought to light, for example, new models of “cleanrooms for lease,” “SME mirroring,” and hospitals spinning out CDMOs and taking on orphan disease programs.
- Quality and Compliance
Is there anybody out there who hasn’t heard of, as phrased in earlier editorial, The Immutable Law? If so, here goes:
Among the three qualities you require from an outsourcing experience – high quality, low price, and fast execution – you can only get a combination of two of them. We depict it this way:
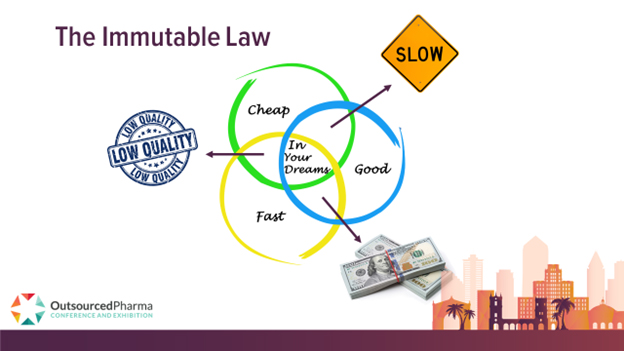
New here is this: Quality, appended now with Compliance, must supersede all other considerations.
Perhaps quality has always been top with many Outsourced Pharma readers. Still, today sponsors need to effectively append Quality and Compliance as a single "must-have" category, but simultaneously and effectively segregate these two when considering outsourcing partners.
Do they have both, are they separately functioning well, and are they joined at the hip.
Today’s factors:
- Innovative scientific pursuits and technologies only enabled by novel equipment and processing
- Platforms of drug discovery increasing the pace of subsequent clinical pursuits
- Simply, more outsourcing and global markets
- Regulatory bodies pumping out new and updated guidance/regulations to keep up with sponsors and science
These all lead to a resolute pursuit of getting the quality right with an eye on all current compliance needs, and evolving circumstances.
Despite desires for leaniality, it requires both internal professionals (or at least trustworthy consultants) as well as personnel at your CDMOs to be up on, and proactive with, the governing regulatory agencies.
New And Old
Fundamentals, by way of definition, are constant. But they are not immutable.
Therefore:
Add “two-way” to communication, insert a higher dose of flexibility and agility, and refocus on quality and compliance.
That appears to me where we need to go after decades of participating in our outsourcing world.
