Unraveling The Complexities In Nasal Vaccine Development, Manufacturing, And Device Selection
By Mark Ignaczak, Director, Innovation and Partnerships - Nasal Delivery, Catalent, Somerset, New Jersey
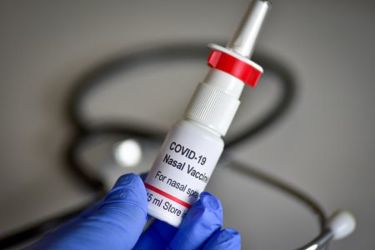
While the needle remains the most common delivery vehicle for vaccines, a large number of patients suffer from needle phobia, making the intranasal delivery route a promising alternative to help boost treatment compliance. The nasal delivery of vaccines is a growing field in terms of investment in research and development resources along with late-phase clinical trials. The challenges of combatting the COVID-19 pandemic supercharged and revitalized vaccine delivery via the intranasal pathway, with more resources being deployed to advance delivery methods.
As you consider developing a nasal delivery program for a vaccine or therapy, a partner with experience of the development and manufacturing techniques that can see a program through small-scale clinical to commercial supply is crucial. Support for technical transfers and scale-up ahead of clinical and commercial launch can ensure development timelines are met and unnecessary costs are avoided. Choosing a development and manufacturing partner with a global presence and expertise in intranasal delivery is essential for product development and commercialization.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Outsourced Pharma? Subscribe today.
