The Regulatory Affairs Function Is Evolving. Are You Evolving With It?
By Maggie Chan, Nora Hernandez, and Hilde Viroux, PA Consulting

The regulatory affairs (RA) function is responsible for the approval and ongoing regulatory compliance of drugs and medical technologies. However, regulatory affairs is typically seen as a cost center and innovation stifler across horizontal functions and executive leadership. Internal stakeholders will mistakenly see RA as a bottleneck to speed to market and revenue generation. This is not true, as RA enables revenue creation and prevents unnecessary expenditures (i.e., recalls, legal fees, and reputational damage). As a result of this perspective, regulatory teams generally lack the baseline funding for effective operations and do not have direct exposure to innovative enterprise AI and machine learning (ML) tools.
The talk around AI has caused many in the space to wonder what its impact will be on their role. What will the future regulatory affairs role look like? What types of tools and skills will determine individual and team success? In this article, we offer insights on what the future of regulatory affairs may entail and how to succeed in that role.
Regulatory Affairs Isn’t A “One Size Fits All” Function
Regulatory affairs is an essential business function within life sciences companies. RA specialists and associates comprise several capabilities that are integral to ensuring compliance with relevant regulations across commercial markets. These capabilities include:
- regulatory strategy
- regulatory authority relationship management
- regulatory submissions & approvals management
- regulatory intelligence
- regulatory operations.
Regulatory affairs specialists have various primary objectives based on their role in the RA function. Some roles in RA are more strategic, while others focus more on execution. A regulatory strategist provides input to development teams regarding current product designs and future ideation. Strategists are the key advisors for predicting which regulatory pathways (e.g., Class I–III) will be viable for a given asset. On the external side, authority relationship managers will establish working relationships with regulatory bodies (i.e., the FDA, competent authorities, notified bodies, etc.) and stay abreast of changing priorities, processes, and leadership from the regulators. Employees who work in regulatory submissions and approvals management maintain in-market product registration for products. Regulatory intelligence officials collaborate with industry peers to influence legislation and identify important trends in legislation and guidelines. Operations staff may coordinate and execute ongoing regulatory operations tasks, including labeling advisories, translations, and more, depending on the organization.
RA personnel tend to have certain educational backgrounds and skillsets. Many are highly technical individuals with bachelor’s and master’s degrees in science, technology, or engineering. To secure a role in RA, a level of familiarity with regulatory guidelines is required as well, depending on role and seniority. Strong written and verbal communication skills are needed to decipher and draft complex documentation. Knowledge of regulatory information management (RIM) systems is also becoming increasingly necessary.
People with RA experience are in high demand due to increasing global regulatory requirements. Authorities, notified bodies, and industry are competing for people with the same profile and RA expertise.
Is Outsourcing Or Insourcing The Best Option For Regulatory Affairs In Your Company?
In order to reduce costs or to enhance the RA function, companies may decide to outsource certain aspects of the RA activities. When deciding between insourcing and outsourcing RA activities, life sciences companies must carefully assess the benefits of each approach.
Outsourcing is often chosen for resource-intensive and time-consuming tasks such as compliance documentation management, publishing and submission (e.g., 510(k), clinical evaluation reports, routine reports), clinical trial management (data collection and reporting), labeling and packaging (including translation), and post-market surveillance (literature review and complaint analysis). By outsourcing these activities to lower-cost centers or third parties, companies can achieve immediate cost benefits, as these vendors usually have the necessary infrastructure in place. Additionally, outsourcing provides access to specialized expertise, enabling companies to navigate international markets without the need for extensive hiring and training. This allows internal resources to focus on strategies for new products, regulatory intelligence, and other core business activities. While outsourcing offers scalability and flexibility to address changing demands, companies must consider the risk of losing know-how due to overreliance on external vendors.
Insourcing offers enhanced data security and cybersecurity by keeping sensitive information within the company, thereby reducing the risk of leaks or data breaches. An in-house RA team ensures a consistent approach to regulatory and quality standards, allowing for closer monitoring and maintaining compliance. This setup also streamlines decision-making processes, fostering efficient governance and improving relationships with regulatory agencies through timely responses. Additionally, an in-house team can implement a more robust cost structure over time, potentially reducing long-term expenses.
How Can AI Aid Regulatory Affairs?
Balancing these factors is crucial for companies aiming to optimize their RA activities effectively. Likewise, artificial intelligence is an emerging technology that also can optimize RA activities. But what is so special about AI? How can it help RA? And how is it different from the tools that are currently implemented?
AI encompasses analytics, automation, and data handling that can help improve RA performance and support executive decision-making.
Analytics: AI can analyze vast amounts of data quickly and accurately, identifying trends and patterns that might be missed by a person. This can help in predicting regulatory changes and assessing their potential impact within the organization. On a day-to-day basis this is identifying new legislation, summarizing relevant information, analyzing public comments and feedback, and producing an impact assessment that the RA team and organization leaders can easily digest to make informed decisions, ensure compliance, and efficiently update standard operating procedures (SOPs).
- Natural Language Processing (NLP): AI’s application of NLP is revolutionizing document analysis. By interpreting complex regulatory documents, AI aids in managing intricate compliance information.
- Predictive Analytics: AI’s foresight in anticipating compliance issues enables organizations to proactively address potential problems, ensuring a smoother regulatory journey.
- Generative AI: With the ability to sift through vast amounts of data, generative AI not only generates precise compliance reports but also suggests customized solutions to stay ahead of regulatory challenges.
Automation: Routine tasks such as data entry, compliance checks, and report generation can be automated, reducing the risk of human error and making the process more efficient. AI can automatically format regulatory documents and extract, categorize, and analyze the information to ensure it is accurate and in compliance with the requirements.
- Automated Submissions: The automation of regulatory document submissions using AI ensures accuracy and consistency, significantly reducing the risk of human error.
- Regulatory Intelligence: AI enhances the agility of regulatory processes by automating updates of SOPs in real time, responding swiftly to regulatory changes.
- Surveillance and Monitoring: AI can automatically search, identify, and refine relevant scientific literature, clinical trial reports, and case studies to support market surveillance activities. AI also can monitor and analyze complaints and new publications to identify trends and alert regulatory teams on emerging safety signals or adverse events.
Data Handling: AI can manage and organize large data sets from multiple sources, ensuring that information is consistent, accurate, easily accessible, and up to date. AI systems can be programmed with rules based on clinical trial protocols, quality inspection, labeling requirements, and several other sets of information to support the data management and analysis. This is crucial for maintaining compliance with regulatory requirements throughout the end-to-end cycle of medical devices.
- Rule-Based Learning: AI employs rule-based learning to decode complex regulations, offering clear and tailored guidelines that align with specific business requirements.
- Efficiency in Document Management: The advancements in AI-driven document management systems are not only increasing efficiency but also streamlining the regulatory processes.
Deployment of these AI capabilities can significantly enhance the dynamics within the medical device companies and lead to more proactive and strategic regulatory management. Additionally, these advancements revamp interactions with regulatory authorities. As AI continues to evolve, it promises to bring about even more streamlined and efficient compliance workflows, ultimately benefiting the entire healthcare ecosystem.
A variety of AI tools are currently available that cater to different aspects of regulatory processes. Some examples can be found in the table below.
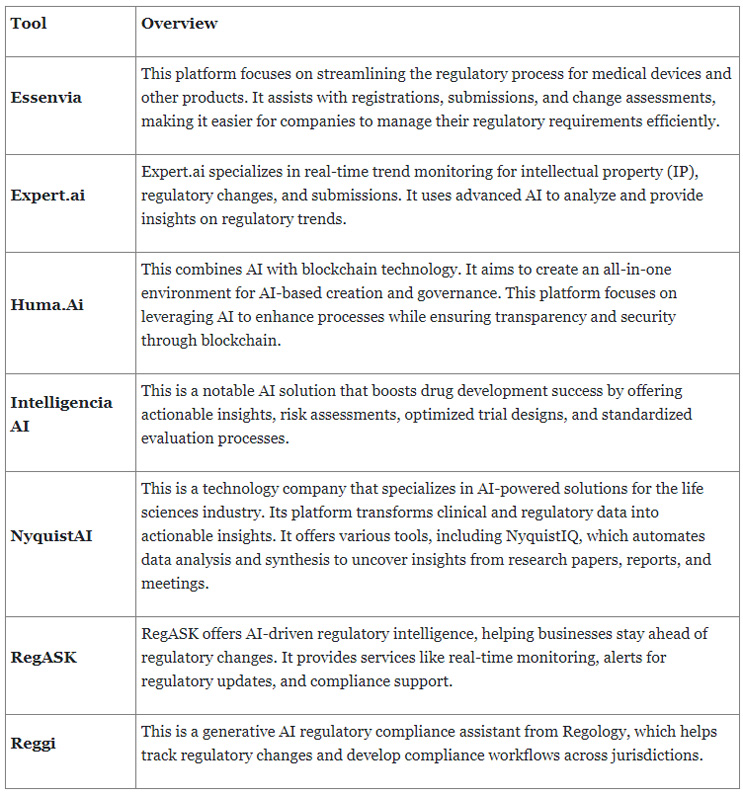
Moreover, AI is leveraged for automated document generation, compliance monitoring, clinical trial design, risk assessment, and predictive analytics, significantly reducing the time and cost associated with these tasks. These tools exemplify the transformative impact of AI in the field, providing regulatory professionals with powerful resources to meet the demands of a complex and dynamic regulatory environment.
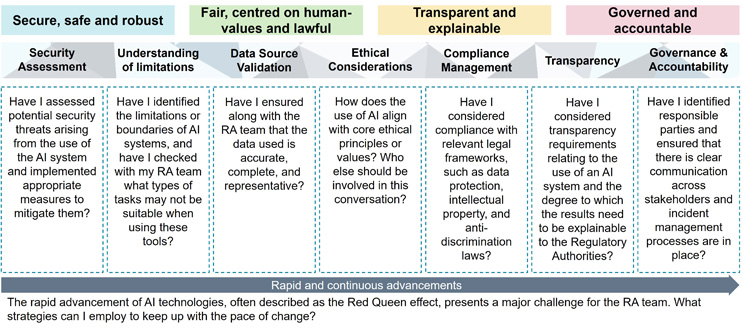
The use of AI in the realm of regulatory affairs presents a complex array of challenges. At PA Consulting, we structure and frame AI risk as shown in the diagram below:
Click on the figure to enlarge.
These challenges underscore the dynamic and ever-changing landscape of AI technology, underscoring the necessity for a regulatory framework that is both flexible and robust, capable of evolving alongside AI advancements while safeguarding safety, effectiveness, and ethical integrity.
In the rapidly evolving landscape for life sciences companies, RA professionals are increasingly required to adapt to the integration of AI in their field. For example, it may require individuals with more data science and computer science backgrounds. These fundamental skills would be necessary to properly interact with models based on NLP and generate material and actionable insights with the help of AI. By allowing digital tools or companions to make quick work of monotonous tasks, RA personnel can focus more time on intelligence and relationship management with internal stakeholders and regulators. Because AI will create a shift in RA roles, responsibilities also will change. RA personnel may become more involved and responsible for project management, data monitoring, and the environment for AI enabled RIM systems.
Conclusion
AI tools can significantly enhance the output and efficiency of the RA function. This may impact the tasks that are outsourced, in the sense that companies will be able to do more in-house. It also impacts the profile of the future RA specialist. Apart from regulatory expertise, an RA professional will have to learn to interact with NLP models and critically interpret outcomes. When planning for investment in AI tools, RA leaders should assess how they will impact their way of working and upskill the RA professionals accordingly.
 About The Authors:
About The Authors:
Maggie Chan is an expert in global medical device regulations at PA Consulting and has supported several transformation projects in the regulatory function.
 Nora Hernandez has extensive experience in change management and continuous improvement at PA Consulting, and her work takes into account the human element of change.
Nora Hernandez has extensive experience in change management and continuous improvement at PA Consulting, and her work takes into account the human element of change.
 Hilde Viroux is a senior regulatory strategist at PA Consulting focusing on new and emerging legislation affecting the regulatory function.
Hilde Viroux is a senior regulatory strategist at PA Consulting focusing on new and emerging legislation affecting the regulatory function.