Marks Took On FDA Vaccine Leadership Position – What Happens Now?
By Nancy Bradish Myers, Catalyst Healthcare Consulting, Inc.

“If you want something done, ask a busy person” is a maxim variously attributed to Benjamin Franklin, Elbert Hubbard, and Lucille Ball. Regardless of its origin, the philosophy certainly has flourished at the FDA during this pandemic. It also has informed the long agency tenure of Acting Commissioner Dr. Janet Woodcock, who more than once has made the choice to juggle dual FDA management positions.
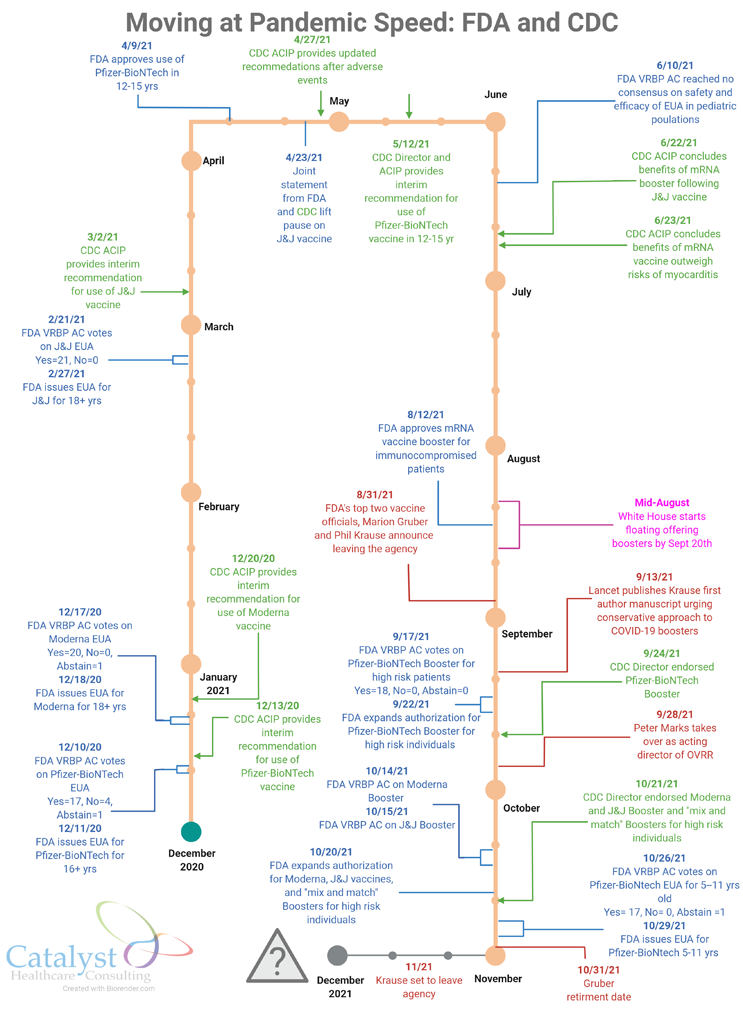
So, it comes as no surprise that under her watch, Center for Biologics Evaluation and Research Director Dr. Peter Marks decided to temporarily take on the leadership of the Office of Vaccine Research and Review after both its director, Marion Gruber, and deputy director, Philip Krause, unexpectedly announced in late August their decisions to exit the FDA.
While Marks’ move to serve as acting director of OVRR initially raised a few eyebrows, there were also palpable signs of relief that the departures of Gruber and Krause wouldn’t result in a sustained leadership gap, and that a strong, steady, and experienced hand would take the wheel while the office’s pandemic work remains in high gear.
Steady Leadership Is Key To Pandemic Response
Examining the reasons why a center director felt the need to step into such an important role at such a critical time may help inform future FDA succession planning efforts. It also may help sensitize lawmakers to the extreme demands on certain centers, especially as they consider CBER’s resource needs in future appropriations rounds and Prescription Drug User Fee Act (PDUFA) VII reauthorization efforts.
Clearly, the unprecedented workload from the pandemic set the scene. With hundreds of thousands of people dying, treatments scarce, and vaccines the best way to stop the spread, OVRR leadership and staff had been put to the task, scientifically and policy- and process-wise.
Even now, the pressure-cooker environment persists for OVRR, and the staff continues to rise to the challenge against a backdrop of uncertainty, urgency, and political pressure.
After speaking with people both inside and outside the agency, I can see that there were no perfect solutions to this situation. My sense is that Marks decided that the office needed someone who could jump into the role and help the staff make the difficult approval and booster calls without a moment’s delay. Since the pandemic became the most important priority for CBER, Marks had been reviewing vaccine files to stay abreast of the science, while at the same time helping his team handle the pandemic workload.
Marks undoubtedly also considered the fact the pandemic is still far from over. Though case and death rates may be easing in some communities, COVID infections continue to surge in others, especially in colder climates. As winter quickly approaches and people are forced to spend more time indoors, the U.S. could see infections spike.
With an ongoing public health crisis of these proportions, it makes sense that Marks chose to jump in to help tackle the immense workload rather than diverting his attention to recruitment, interviewing, and training. Most FDA watchers know that agency onboarding is notoriously slow. And while some have argued that there are internal staffers who could have been elevated, it’s also likely that doing so would have created even larger holes in the expertise needed to address OVRR’s enormous and urgent to-do list.
Marks’ choice to take on OVRR will help ensure timelines for vaccines and boosters do not slip. Additionally, by plugging the OVRR leadership gap, he will serve as a buffer to shield staff from direct pressure from the outside to do more, faster.
The decision may be seen by some as paternalistic, but I think Marks’ willingness to continue putting his own reputation on the line as the agency must make highly scrutinized vaccine decisions may be exactly what the scientific staff needed. Improving staff morale has always been a focus of Marks, and he undoubtedly is mindful of the growing competition for CBER talent outside the agency at a time when the staff continues to be burdened by the demands of the pandemic.
And, of course, there is precedent from the top for Marks’ decision to take on OVRR. As noted, Woodcock straddled dual roles while she was Center for Drug Evaluation and Research Director – first running the Office of New Drugs and then the Office of Pharmaceutical Quality – as she reorganized the functions.
It’s unclear how long this dual role will last and whether it will slow progress in other important areas CBER oversees. I expect Marks will likely wear both the CBER and OVRR director hats through the end of this year, with the OVRR director and deputy director searches beginning in earnest after Gruber and Krause leave the agency. Gruber retired Oct. 31, and Krause will depart sometime this month (November 2021).
The search to fill these key leadership positions will probably start internally, with a focus initially on the deputy director spot. There likely will be a recognition that FDAers who have worked through the pandemic have been fire-tested and have absorbed lessons that can’t be found in any textbook or standard operating procedures.
Staffing Challenges Remain
There are a couple of cautionary tales in the unexpected retirements of Gruber and Krause.
As the pandemic continues, the Biden Administration and Congress must be careful not to get out in front of the critical and capable agencies that are doing the hard work to contain the pandemic. Putting too much pressure on scientists and regulators can have deleterious staffing implications at a time when expertise and efficiency are most needed. Overt political pressure and allowing others to speak for FDA also can and will undermine the credibility of an agency.
Though no government agency can prepare for all potential staffing situations – especially losing two critical staffers with little warning during a pandemic – FDA management needs to be even more mindful of succession planning, by anticipating retirements and building a solid management bench to the extent possible. With many agency leaders at or close to retirement age, succession planning has been a constant challenge for FDA’s Office of Human Capital Management.
It is noteworthy that CBER will have an unprecedented staffing expansion opportunity if and when Congress authorizes PDUFA VII in Q3 2022. Given the chronically under-resourcing – some might say underappreciation – of the center, PDUFA VII negotiators agreed to increase CBER’s budget by $348.7 million over five years, which is the equivalent of adding 233 new full-time equivalents. The bulk of the hiring goal is set for 2023.
Though PDUFA targets new resources and headcount for initiatives agreed upon by FDA and industry negotiators, the influx will offer CBER leadership an opportunity to reevaluate levels of center staffing as a whole, including succession planning.
As CBER works to recruit additional talent and conduct succession planning, we all must acknowledge the current tight labor market for experts in the fields of vaccines, biologics, and cell and gene therapies. Not only are more companies focused on vaccine development, but there is a distinct development tsunami hoovering up candidates. This may make it more difficult for CBER to recruit as it attempts to grow its very competent workforce with more constrained compensation and benefit levels than the private sector.
Taking all these factors into account, Marks’ decision to leap into the OVRR leadership void was an admirable but unsurprising choice from a dedicated leader. I just hope he will have an opportunity to use up those accrued vacation days — he deserves it!
About The Author:
 Nancy Bradish Myers, JD, founder and CEO of Catalyst Healthcare Consulting, Inc., is a Washington, D.C.-based attorney with deep expertise in healthcare law and regulation, policy development, patient engagement, and government relations. She served as senior policy counsel in the Office of the FDA Commissioner, as well as special assistant/senior strategic advisor to the FDA acting deputy commissioner for operations. Myers has been closely involved in drug, biotechnology, and medtech regulatory issues for over 25 years. She currently advises cutting-edge clients on regulatory and health policy matters. You can reach her at nancy@catalysthcc.com or connect with her on LinkedIn.
Nancy Bradish Myers, JD, founder and CEO of Catalyst Healthcare Consulting, Inc., is a Washington, D.C.-based attorney with deep expertise in healthcare law and regulation, policy development, patient engagement, and government relations. She served as senior policy counsel in the Office of the FDA Commissioner, as well as special assistant/senior strategic advisor to the FDA acting deputy commissioner for operations. Myers has been closely involved in drug, biotechnology, and medtech regulatory issues for over 25 years. She currently advises cutting-edge clients on regulatory and health policy matters. You can reach her at nancy@catalysthcc.com or connect with her on LinkedIn.
