Intensifying Downstream Processing With Magnetic Separation
By Julian Galbusera, Ines Zimmermann, and Paula Fraga-García, Technical University of Munich

Biopharmaceuticals and recombinant proteins for therapeutic, diagnostic, or industrial applications are on the rise due to their high selectivity and effectivity, including several antibody classes, therapeutic enzymes, polynucleotides for vaccines, gene therapeutics, hormones, and growth factors. These diverse products bring increasingly complex manufacturing profiles in which unit operations include more steps requiring a higher degree of skill. Here, standard biomanufacturing downstream processing (DSP) procedures face cost and productivity limitations, very often caused by the prevalent chromatography operations. As illustrated in Figure 1A, conventional packed-bed chromatography consists of relatively large particles (low back-pressure) with high porosity (high specific surface area), through which feed streams and buffers pass, whereby target molecules and impurities interact with the particles differently.1 For instance, in protein A chromatography used for mAb capture, mAbs selectively bind to protein A ligands on the beads (loading), then impurities are washed off the column before the mAbs are desorbed using a low-pH buffer (elution), and the column is regenerated for renewed loading (see Figure 1B).2
Although conventional packed-bed chromatography is well established, it requires extensive feed clarification to prevent column clogging.3 Furthermore, the diffusive mass transfer in the porous structure is slow, and the effective surface area is reduced due to the steric hindrances of large biopharmaceutic modalities.4
Biologics are more complex and larger in size than conventional small molecules, making these two factors increasingly limiting. Thus, alternative separation techniques are essential to overcome these bottlenecks. Additionally, process intensification offers great opportunities to enhance bioprocessing. Process intensification is the combination of integrated process design, novel technologies, and equipment to enhance overall productivity by reducing production time, improving product quality, and minimizing resource use. Magnetic separation based on functional magnetic particles is a highly promising technique for process intensification, as we will demonstrate in this article.
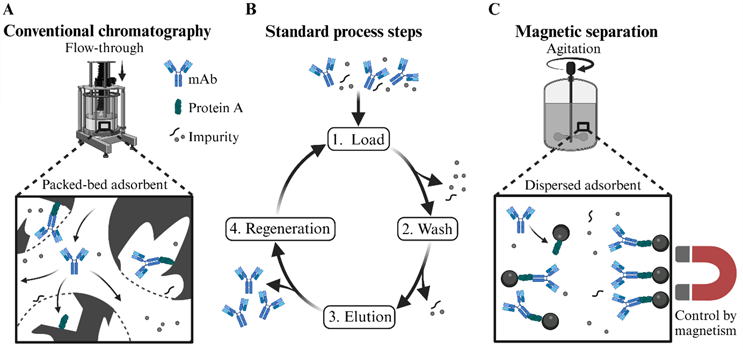
Figure 1. Schematic illustration of the conventional diffusion-limited packed-bed chromatography and the novel magnetic separation principle. Both techniques are depicted for purifying mAbs using protein A-based ligands. (A) In conventional chromatography, a packed bed of functional resin beads is used through which the feed stream flows. In contrast, (C) magnetic separation relies on functional magnetic particles (MPs) that are agitated within the feed stream and can be magnetically retained for buffer exchanges. (B) Standard steps in both techniques include binding mAbs to protein A ligands, washing away impurities, eluting mAbs, and regenerating the matrix.
Magnetic Separation Is A Novel DSP Technique
Magnetic separation relies on the separation of magnetic and non-magnetic materials. Traditionally, it has been applied in fields such as mineral beneficiation, steel production, and wastewater treatment to separate substances with intrinsic magnetization.5,6 Furthermore, it is well-established in laboratories for analytics and diagnostics, partly on automated high-throughput platforms.7
In the last 10 to 15 years, the magnetic separation technique has also gained popularity in preparative bioseparation (see Figure 1C).8 Biologic materials are thereby selectively bound to magnetic particles (MPs), which can, for instance, be produced in simple and cheap co-precipitation reactions or from natural sources.9 MPs usually have a size between 10 nm to 100 µm and consist of magnetic cores (e.g., iron oxide), which can be modified like in chromatography with application-specific chemical or biochemical coatings (e.g., functional silanes or saccharide-based biopolymers) and/or biological affinity ligands (e.g., protein A). Magnetic separation generally follows the same steps as chromatography (loading, washing, elution, and particle regeneration, illustrated in Figure 1B).
However, unlike the flow-through columns in chromatography, the MPs are dispersed and agitated in their respective feed streams/buffers, and buffer exchanges are conducted by magnetically retaining the particles and replacing the fluid (see Figure 1C). The general non-porosity resulting from the dispersed particle adsorbent brings two decisive advantages compared to standard chromatography: enhanced mass transfer of large modalities and the potential processing of unclarified feeds. Furthermore, MPs can have high target-binding capacities, which is important for efficient processing.
Magnetic Separation Has A High Potential For Downstream Intensification
Every magnetic separation process generally consists of three main components that can be adapted accordingly:
- the magnetic particle,
- its surface functionalization, and
- the magnetic separation device.
While the choice of separator is relatively straightforward due to the limited number of available systems for biopharmaceutical applications, the feed stream and the final goal influence the choice of MPs and their functionalization. In the following, a few use cases will be discussed that propose different choices for the three components (Figure 2).
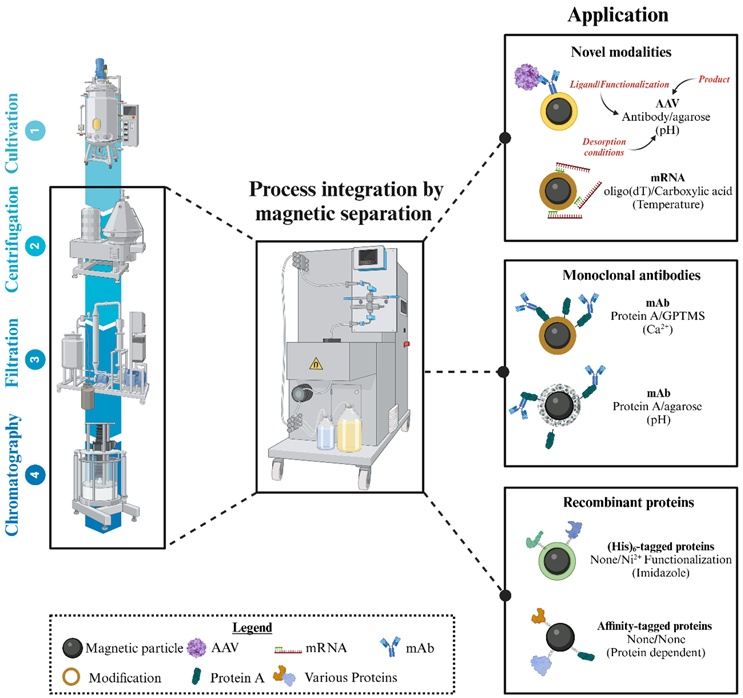
Figure 2. Simplified schematic illustration exemplifying selected reported magnetic separation studies for the purification of novel modalities, mAbs, and recombinant proteins. MPs can be modified with specific functional groups or ligands to modulate the binding specificity of the target. The key benefit of the magnetic separation technique is the potential integration of clarification and capture steps that are usually performed by costly and time-consuming centrifugation, filtration, and chromatography unit operations.
Purification Of Monoclonal Antibodies
The intensification of upstream processes (USP) in mAb production has led to significantly higher titers through perfusion and high cell density fermentation methods. Consequently, the DSP now faces the challenge of handling two distinct starting situations.
- Small volumes of high cell density broths with high titers (fed-batch) or
- Large volumes with low mAb titers but no cells (perfusion).
Centrifugation and filtration operations must process much higher cell concentrations, while chromatographic steps must cope with extremely high volumes or highly concentrated loads. This poses a significant challenge for traditional purification methods and accentuates the necessity for a better alignment between USP and DSP even further.
Magnetic separation offers a potential solution for these two problems. For high cell density processes, the integration of the three process steps of centrifugation, filtration, and chromatography in a single separation step can solve the issue of high solid loads. In contrast, for perfusion cultures, the absence of a stationary phase means volume-independent adsorption and thus significantly less processing time.
The unique properties of magnetic particles facilitate the integration and open a platform of materials that can be tailor-made, with increasing complexity, if the final goal requires it. Their high adaptability enables the immobilization of traditional and innovative protein A ligands known from chromatography through covalent or non-covalent techniques. Subsequently, the protein A functionalized MPs can be directly added to the cell broth or cell culture supernatant.
Here, the second useful characteristic comes into play: their reduced or almost negligible porosity compared to traditional chromatographic resins, as mentioned before. Several case studies are presented based on this working principle, although with slightly different approaches.
Integration Of Clarification And Capture Yields High Purities
In practice, the protein A-functionalized MPs are directly added to the cell culture broth. Even state-of-the-art cell densities of up to 100 x 106 cells mL-1 do not adversely affect the binding or elution behavior, as Brechmann et al. showed using protein A-loaded agarose MPs.10, 11 The purification of approximately 16 L of CHO cell culture, with a titer between 1.3 and 1.5 g L−1 IgG, took five to eight hours. The saturation of the binding sites on the particles was thereby completed within one hour of incubation. With the integrated processing, they achieved very low host cell protein (HCP) levels in the eluates (< 5 ppm), which they attributed to the gentle processing without notable cell lysis.11
Ebeler et al. demonstrated the direct mAb capturability from cell culture using the GMP-compliant rotor-stator high-gradient magnetic separator (RS-HGMS) system MES 100 RS by Andritz KMPT GmbH (Germany).12 They reported a 3-times higher process productivity per mL of applied resin than that of conventional chromatography, which highlights the great potential of the magnetic separation technique.
Shortcomings And Bottlenecks
Processes using agarose-coated MPs can compete with chromatographic processes, but are limited by their rather slow kinetics that result from their porosity. To address this, our group developed an alternative particle approach based on non-functionalized and non-porous MPs with a peptide-tag-promoted affinity ligand immobilization.13 This method eliminates time-consuming and costly particle coatings, reduces chemical consumption, and enables fast IgG interaction within minutes.
Another drawback of conventional MPs for mAb separation is the requirement of acidic pH conditions, which can be detrimental to mAb products. We addressed this issue by covalently coupling an engineered calcium-dependent protein A ligand to GPTMS functionalized MPs.14 This strategy offers faster kinetics caused by the largely non-porous particles and enables gentle elution conditions around pH 5.5.
However, the main challenges that need to be addressed are the low concentration factors and the lack of suitable, large-scale separator systems. For instance, processing the supernatant of a 2,000 L bioreactor with a mAb titer of 5 g L-1 (10 kg in total) would require approximately 400 batch runs, assuming the processing of 25 g mAb per cycle.15 Even with short cycle times of one to two hours, the sheer number of runs presents a bottleneck, largely resulting from the limited filter capacities of current magnetic separators. Two strategies are proposed for this problem. First, magnetic separation-based processes can integrate so well that clarification and filtration steps may be skipped. The time saved through this can compensate for some of the high cycle numbers.
Secondly, the development of large-scale magnetic separators with particle capacities of several hundred grams to kilograms is of fundamental importance. As a start, an RS-HGMS with a 10 L chamber is available by Andritz KMPT GmbH, which would theoretically decrease the number of cycles to approximately 40. In combination with the development of multi-scale models of magnetic separators, as recently described by Tesanovic et al.,16 a scale-up would become more straightforward.
Based on these models, the chamber and matrix design can be optimized in-silico and adapted accordingly. It should also be considered that elution at increasing particle concentration enables higher potential product concentrations, and the implementation of an additional smaller elution chamber could thus be promising, as reported by Brechmann et al.10 In conjunction with improved separator design, continuous or semi-continuous processing should be targeted, e.g., by serial connection of separators or magnetic centrifugation. Semi- or fully continuous processing would likely significantly increase productivity, as it has done for conventional chromatography.17
Purification Of Novel Modalities
While mAb processes benefit from established platforms, novel modalities such as virus-based treatments, plasmid, RNA-based therapeutics, and CAR T-cell therapies are still in the early stages of development. The complexity of purifying these molecules is often hindered by low productivity, which drives up costs and delays market readiness.18 Process development for these novel modalities is still in its infancy. This makes it an ideal time to implement magnetic separation in the process development to facilitate later implementation in GMP-compliant processes.
Turco et al. purified recombinant adeno-associated virus (rAAV) from crude HEK293T cell lysate using the RS-HGMS (Andritz KMPT GmbH) system with Mag Sepharose beads functionalized with rAAV-specific ligands. Their process achieved 63% recovery with significant reductions in HCP and DNA while improving productivity tenfold compared to chromatographic-based processing.18
Magnetic separation is a well-established method for DNA purification at an analytical scale. Therefore, it is not surprising that it is also investigated for the large-scale purification of mRNA, especially concerning the potential of mRNA-based vaccines.
The use of magnetic beads functionalized with oligo(dT) in a 3D-printed, single-use magnetic separation chamber proposes an interesting approach to a biopharmaceutical application.19 While large-scale examples are lacking, magnetic separation may be promising for the purification of CAR T-cells, as recently proposed by Harrer et al.20 As most of these molecules require gentle handling, the impact of physical stress caused by the particles must be investigated. It, therefore, remains unclear whether the developed large-scale magnetic separators can be used to process novel modalities without further modifications. As already described for the purification of mAb processing, the lack of large-scale magnetic separators is a bottleneck for further process implementation in production scale.
Purification Of Recombinant Proteins
Purifying biopharmaceutical proteins from microbial lysates, fermentation broths, or blood serum differs from the above-described use cases as they typically involve low-price molecules. Therefore, simple MPs without ligands or functionalizations are desirable for reducing costs.
Here, separation yield and purity can be tuned by the correct choice of surface area-to-biomass ratios. In the end, it remains a gamble between specificity and cost-effectiveness.
Short affinity peptide tags fused to the protein of interest can be used to achieve some specificity when using non-functionalized MP. The result is a process based on an extremely low-cost, non-toxic MP with high binding capacity although reduced specificity compared to functionalized MP.21
We believe their application for in-situ product removal from fermentation broths with an extracellular product of interest has enormous potential. MPs with immobilized metal ions such as Cu2+ are a good choice for higher specificity and still low material cost.22 This approach combines the benefits of the in chromatography frequently applied (His)6-tag with the benefits of magnetic separation. It is worth mentioning that the know-how from chromatography processing can be widely applied to tune magnetic separation, as both are adsorption-based unitary operations. Here, magnetic separation suffers from a low degree of research and technical development but benefits of design flexibility and no mass transfer limitations.
Large-scale processes for the purification of proteins using low-cost or non-functionalized MPs in the literature revealed yields and purities comparable to reference chromatography processes. Once again, their main benefit is their significantly shorter process time, especially when the centrifugation or filtration steps can be omitted. Nevertheless, the same downsides as described for the processing of mAb remain. The aspect of missing large-scale devices is for recombinant protein recovery even more serious, as these molecules are typically produced at even higher volumes, further increasing the number of cycles needed using the small-scale apparatus currently available on the market.
Future Perspectives
Biopharmaceutical manufacturing is stiff, with finely tuned and optimized parameters within narrow windows, as is the case of conventional chromatography. Thus, limited improvements are expected in the future, and real process intensification demands disruptive techniques – the magnetic separation technique has great potential, as we demonstrated above. However, further development of this technique is required, as commercially available batch separators significantly constrain the throughput and the achievable concentration factors. In our opinion, separators with enhanced particle capacity and (semi)-continuous magnetic separation modes are key for higher throughput and productivity and should thus be the focus of future research and development.
Our group already works on the implementation of process analytical technology (PAT) and the development of modeling approaches for real-time process control that are decisive for continuous applications.16 Moreover, the development of novel and more efficient functional particles should be one more research focus, as the particles notably influence the applicability and productivity of magnetic separation processes. The efficient production of these particles through automated integration of the MP synthesis and their application in bioseparation applications is required to lower costs and holistically increase process productivity. Lastly, we want to address the single-use equipment trend in the biopharmaceutical industry, which could be approached with single-use inlets in the magnetic separation chamber, as demonstrated by Wommer et al.23
In our opinion, the three most decisive advantages of the magnetic separation technique are
- the fast interaction of large amounts of target molecules with the magnetic particles due to notably reduced mass transfer limitations and high binding capacities,
- the capability of integrating centrifugation, filtration, and clarification steps, which enables high-level process integration, and
- the cost-efficient and simple large-scale synthesis of bare magnetic particles.
However, we are aware that magnetic separation is still in its infancy, and it is challenging for novel technologies to gain acceptance in the strictly regulated biopharmaceutical industry and compete with established techniques. Nevertheless, we are confident that magnetic separation offers a valuable approach that can pave the way to improved overall productivity, enhanced sustainability, simplified processing, and time saved.
Editor's note: Galbusera and Zimmermann contributed equally.
References:
- G. Carta, A. Jungbauer, Protein Chromatography, Wiley, 2020.
- C.L. Gaughan, The present state of the art in expression, production and characterization of monoclonal antibodies, Mol. Divers. 20 (2016) 255–270. https://doi.org/10.1007/s11030-015-9625-z.
- A.A. Shukla, L.S. Wolfe, S.S. Mostafa, C. Norman, Evolving trends in mAb production processes, Bioeng. Transl. Med. 2 (2017) 58–69. https://doi.org/10.1002/btm2.10061.
- K.M. Łącki, Introduction to preparative protein chromatography, in: Biopharmaceutical Processing, Elsevier, 2018, pp. 319–366.
- C.T. Yavuz, J.T. Mayo, W.W. Yu, A. Prakash, J.C. Falkner, S. Yean, L. Cong, H.J. Shipley, A. Kan, M. Tomson, D. Natelson, V.L. Colvin, Low-field magnetic separation of monodisperse Fe3O4 nanocrystals, Science 314 (2006) 964–967. https://doi.org/10.1126/science.1131475.
- J. Oberteuffer, Magnetic separation: A review of principles, devices, and applications, IEEE Trans. Magn. 10 (1974) 223–238. https://doi.org/10.1109/TMAG.1974.1058315.
- S. Berensmeier, Magnetic particles for the separation and purification of nucleic acids, Appl. Microbiol. Biotechnol. 73 (2006) 495–504. https://doi.org/10.1007/s00253-006-0675-0.
- S.P. Schwaminger, P. Fraga-García, M. Eigenfeld, T.M. Becker, S. Berensmeier, Magnetic separation in bioprocessing beyond the analytical scale: from biotechnology to the food industry, Front. Bioeng. Biotechnol. 7 (2019) 233. https://doi.org/10.3389/fbioe.2019.00233.
- H.-C. Roth, S.P. Schwaminger, M. Schindler, F.E. Wagner, S. Berensmeier, Influencing factors in the CO-precipitation process of superparamagnetic iron oxide nano particles: A model based study, Journal of Magnetism and Magnetic Materials 377 (2015) 81–89. https://doi.org/10.1016/j.jmmm.2014.10.074.
- N.A. Brechmann, P.-O. Eriksson, K. Eriksson, S. Oscarsson, J. Buijs, A. Shokri, G. Hjälm, V. Chotteau, Pilot-scale process for magnetic bead purification of antibodies directly from non-clarified CHO cell culture, Biotechnol. Prog. 35 (2019) e2775. https://doi.org/10.1002/btpr.2775.
- N.A. Brechmann, H. Schwarz, P.-O. Eriksson, K. Eriksson, A. Shokri, V. Chotteau, Antibody capture process based on magnetic beads from very high cell density suspension, Biotechnol. Bioeng. 118 (2021) 3499–3510. https://doi.org/10.1002/bit.27776.
- M. Ebeler, O. Lind, N. Norrman, R. Palmgren, M. Franzreb, One-step integrated clarification and purification of a monoclonal antibody using Protein A Mag Sepharose beads and a cGMP-compliant high-gradient magnetic separator, N. Biotechnol. 42 (2018) 48–55. https://doi.org/10.1016/j.nbt.2018.02.007.
- I. Zimmermann, Y. Kaveh-Baghbaderani, F. Eilts, N. Kohn, P. Fraga-García, S. Berensmeier, Direct affinity ligand immobilization onto bare iron oxide nanoparticles enables efficient magnetic separation of antibodies, ACS Appl. Bio Mater. 7 (2024) 3942–3952. https://doi.org/10.1021/acsabm.4c00280.
- I. Zimmermann, F. Eilts, A.-S. Galler, J. Bayer, S. Hober, S. Berensmeier, Immobilizing calcium-dependent affinity ligand onto iron oxide nanoparticles for mild magnetic mAb separation, Biotechnology Reports (2024) e00864. https://doi.org/10.1016/j.btre.2024.e00864.
- B. Kelley, The history and potential future of monoclonal antibody therapeutics development and manufacturing in four eras, mAbs 16 (2024) 2373330. https://doi.org/10.1080/19420862.2024.2373330.
- M. Tesanovic, J.P. de Souza, M.Z. Bazant, S. Berensmeier, 2025. Magnetic particle capture in high‐gradient magnetic separation: A theoretical and experimental study. AIChE Journal, e18733. https://doi.org/10.1002/aic.18733.
- L. Gerstweiler, J. Bi, A.P. Middelberg, Continuous downstream bioprocessing for intensified manufacture of biopharmaceuticals and antibodies, Chemical Engineering Science 231 (2021) 116272. https://doi.org/10.1016/j.ces.2020.116272.
- F. Turco, A. Wegelius, O. Lind, N. Norrman, A.-C. Magnusson, C. Sund-Lundström, B. Norén, J. Hedberg, R. Palmgren, Combined clarification and affinity capture using magnetic resin enables efficient separation of rAAV5 from cell lysate, Mol. Ther. Methods Clin. Dev. 30
- L. Wommer, W. Soerjawinata, R. Ulber, P. Kampeis, Agglomeration behaviour of magnetic microparticles during separation and recycling processes in mRNA purification, Eng. Life Sci. 21 (2021) 558–572. https://doi.org/10.1002/elsc.202000112.
- D.C. Harrer, S.-S. Li, M. Kaljanac, V. Bezler, M. Barden, H. Pan, W. Herr, H. Abken, Magnetic CAR T cell purification using an anti-G4S linker antibody, Journal of Immunological Methods 528 (2024) 113667. https://doi.org/10.1016/j.jim.2024.113667.
- S.P. Schwaminger, P. Fraga-García, S.A. Blank-Shim, T. Straub, M. Haslbeck, F. Muraca, K.A. Dawson, S. Berensmeier, Magnetic one-step purification of His-tagged protein by bare iron oxide nanoparticles, ACS Omega 4 (2019) 3790–3799. https://doi.org/10.1021/acsomega.8b03348.
- P. Fraga García, M. Brammen, M. Wolf, S. Reinlein, M. Freiherr von Roman, S. Berensmeier, High-gradient magnetic separation for technical scale protein recovery using low cost magnetic nanoparticles, Separation and Purification Technology 150 (2015) 29–36. https://doi.org/10.1016/j.seppur.2015.06.024.
- L. Wommer, P. Meiers, I. Kockler, R. Ulber, P. Kampeis, Development of a 3D‐printed single‐use separation chamber for use in mRNA‐based vaccine production with magnetic microparticles, Eng. Life Sci. (2021). https://doi.org/10.1002/elsc.202000120.
 About The Authors:
About The Authors:
Julian Galbusera, M.Sc., is a Ph.D. student at the Technical University of Munich at the Chair of Bioseparation Engineering. His research focuses on the implementation of process intensification in microbial production processes through application of novel process concepts like magnetic separation.
 Ines Zimmermann, M.Sc., is a Ph.D. student at the Technical University of Munich at the Chair of Bioseparation Engineering. Her research focuses on magnetic separation concepts for antibodies as an alternative to Protein A-based downstream processing platforms.
Ines Zimmermann, M.Sc., is a Ph.D. student at the Technical University of Munich at the Chair of Bioseparation Engineering. Her research focuses on magnetic separation concepts for antibodies as an alternative to Protein A-based downstream processing platforms.
 Dr. Paula Fraga-García is a senior research scientist at the Technical University of Munich at the Chair of Bioseparation Engineering. Her work includes the study of biomolecule separation from model and complex biological mixtures by magnetic nanoparticles, and electrosorption-based recovery techniques.
Dr. Paula Fraga-García is a senior research scientist at the Technical University of Munich at the Chair of Bioseparation Engineering. Her work includes the study of biomolecule separation from model and complex biological mixtures by magnetic nanoparticles, and electrosorption-based recovery techniques.
