Innovation Advances In mRNA Medicine
By Khaled Yamout and Deborah Day Barbara, The Alliance for mRNA Medicines

The advent of the COVID-19 vaccines served as a catalyst, propelling mRNA into the spotlight. It challenged mRNA innovators to scale out the manufacturing process and provided the new quality standards required for regulatory approval.
Four years later COVID is endemic, and we stand at an exciting juncture. Echoing the FDA’s Peter Marks’ sentiment from the Alliance for mRNA Medicines’ April 2024 Regulatory Summit, “We are at the beginning of the beginning.” The question on everyone’s mind is, “What is next for post-pandemic mRNA?”
As we argued in a previous Advancing RNA column, mRNA has emerged as the fourth pillar of pharmaceutical innovation, intervention, and investment.1 The pre-pandemic mRNA landscape was predominantly based on mRNA derived by in vitro transcription and delivered by encapsulation with known LNP delivery platforms. However, the field is advancing quickly as we witness the evolution of mRNA molecule design, leading to potentially more durable responses and to reduce dosage requirements with the potential of creating safer and more efficacious drugs in a significantly broader array of therapeutic applications. The surge of innovation is substantial, having progressed from fewer than 100 molecules in 2019 to 1,340 molecules in the mRNA discovery and clinical pipelines as of June 2024.
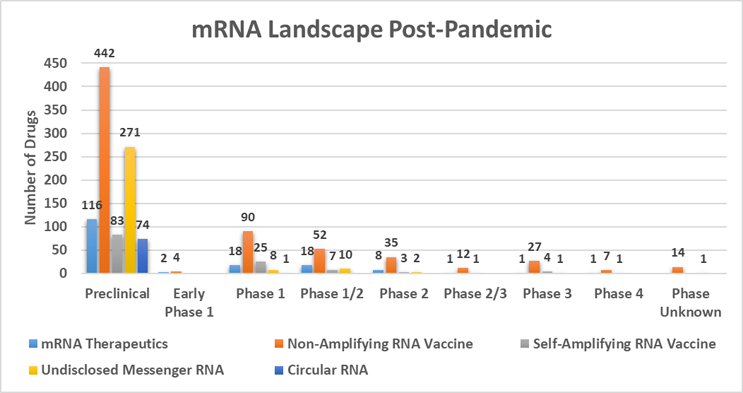
Data provided by Beacon Insights
The mRNA molecules landscape is categorized into three main segments:
- 63% non-amplifying mRNA
- 9% self-amplifying mRNA
- 5% circRNA
While 22% of the molecules in the pipeline are “undisclosed mRNA,” the majority (>92%) of these molecules are preclinical.
The post-pandemic pipeline surge is marked by a fourteen-fold increase in molecules which has led to a substantial investment in innovation. With the formation of 63 new companies and a 53% uptick in the number of financing deals since 2020, it’s clear investor interest and confidence have been piqued. The disclosure of Series A and B funding rounds by companies specializing in mRNA, saRNA, and circRNA between 2020 and 2024 indicates the industry’s concerted effort to transition preclinical assets to clinical stages and to diversify portfolios. With most of the mRNA-focused companies announcing Series A as their latest funding milestone, we can anticipate sustained momentum in the development of these therapies.2
In the following sections, we will review the current and next-gen forms of mRNA, highlighting current progress with/toward each modality to-date.
Nonamplifying mRNA (mRNA)-Based Vaccines And Pharmaceuticals: What We Know
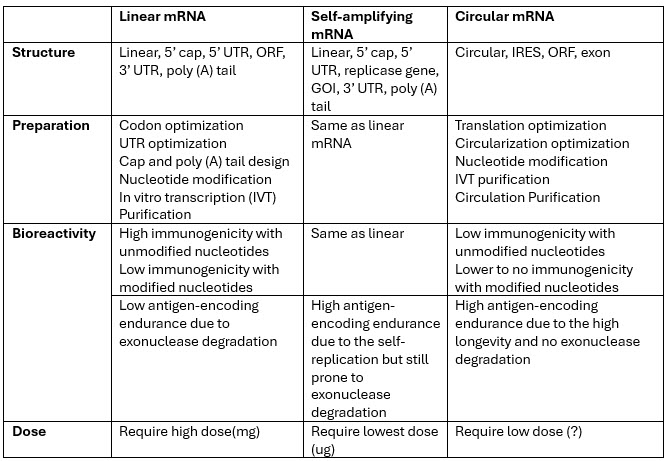
In the earliest biology classes, we learned of the central dogma: DNA (the cell’s hardware) is transcribed into mRNA (the cell’s software) followed by transcription into protein. In vitro transcribed mRNA is the foundation for nonamplified mRNA-based vaccines and pharmaceuticals. mRNA is composed of four main elements: 5’ cap, 5’ and 3’ untranslated regions (UTRs), coding sequencing, and 3’ poly(A) tails. Each of these components serves a specific function and represents a critical quality attribute (CQA) that will define the mRNA’s ability to express the gene of interest in an efficient manner.
CQAs include 5′ capping efficiency and structure; UTR and open reading frame (ORF) structure, length, and regulatory elements; modification of coding sequence; poly (A) tail properties; and mRNA and process purity and impurities. Analytical methods to assess these CQAs have been developed and continue to evolve to address the size of these molecules to be more quantitative, specific, and accurate. It is important to realize that the size of mRNA is roughly nine times the molecular weight of the protein that it encodes since each amino acid corresponds to a trinucleotide codon. The mRNA size, polarity, charge, secondary structure, and the many specialized regions contained within its sequence pose many challenges in analyzing and characterizing mRNA. In most cases the mRNA must be fragmented before analysis, which introduces another variable in the analytical methods.
As a result of collaboration between industry and the United States Pharmacopeia (USP), the USP has published "Analytical Procedures for mRNA Vaccine Quality (Draft Guidelines)”. This document provides guidance on product quality attributes and analytical procedures necessary to ensure the quality of mRNA vaccines. Furthermore, USP recently formed an expert panel on mRNA vaccines to draft a chapter on best practices and to advise on needed standards and tools. In addition, there have been major efforts to advance and introduce new analytical methodologies that are being conducted by analytical labs and providers.3
The understanding of mRNA’s foundational biology and the associated CQAs allowed innovators to move at “warp speed” when designing the first COVID mRNA vaccines. As mRNA molecule innovation advances, we start to solve for challenges of lower doses and durability to make safer and more efficacious vaccines as well as to broaden the potential therapeutic index for new mRNA-based pharmaceuticals. These new medicines will likely deliver a new/expanded set of critical quality attributes that will need to be characterized and understood to achieve successful regulatory approval.
Self-Amplifying RNA (saRNA)-Based Vaccines And Pharmaceuticals: What We’re Learning Today
saRNA molecules are significantly enhanced versions of a conventional mRNA molecule. In addition to the coding sequence for expressed genes, there is an additional viral gene encoding a replicase. The replicase allows the saRNA to make multiple copies of itself, resulting in a lower administered dose and enhanced mRNA vaccine or therapy durability. Proof of this innovation’s promise was seen in late November 2023 when Japan's Ministry of Health, Labour and Welfare approved the first saRNA vaccine developed by CSL and Arcturus Therapeutics known as ARCT-154 for prophylactic treatment of COVID in adults. In trials, 5 ug of ARCT-154 saRNA as a fourth booster showed higher antibody levels and greater durability than 30 ug of Comirnaty, with more participants responding and maintaining elevated antibodies after one month.4,5
It’s important to note that saRNA is more than two to four times the size of the current mRNA used in the COVID vaccine. This represents certain challenges in analyzing the mRNA for purity and impurity profiling due to its size. While most of the analytical methods developed for nonamplifying mRNA can be applied to characterize and analyze saRNA, it still behooves the industry to emphasize the importance of and work with regulators and standards bodies to develop analytical guidelines specific to saRNA and circRNA.
Circular RNA (circRNA)-Based Vaccines And Pharmaceuticals: What We Still Don’t Know (Yet)
One of the newest innovations in mRNA design is circRNA. circRNA is a single-stranded RNA that forms a covalently closed continuous loop, unlike linear mRNA and saRNA, which has free 5’ and 3’ ends. These molecules have significantly different structures than mRNA and saRNA as they have no 5’ caps and 3’poly (A) tails. The circular structure makes circRNA more stable and resistant to degradation compared to linear mRNA and linear saRNA.
circRNA represents completely different analytical challenges due to its different structure and the process impurities associated with its manufacturing process. As such, new analytical methods need to be developed for the characterization and analysis of circRNA, and the CQAs will need to be reevaluated. (For example, capping and poly (A) tail analysis, which we analyze with linear mRNA and saRNA, do not apply to circRNA.) Likewise, methods to analyze the process impurities associated with circRNA production will need to be evaluated to ensure their applicability to this early-stage RNA technology.
Summary
To advance mRNA medicine, it’s essential to cultivate a collaborative ecosystem among industry, academic institutions, and global regulators. As innovation in the mRNA field expands into new molecule designs, chemistries, and delivery methods, we will likely see regulators require understanding of additional CQAs to achieve licensure.
The Alliance for mRNA Medicines (AMM) is a leading global organization dedicated to advancing and advocating for mRNA and next-generation encoding RNA therapeutics and vaccines. The members of AMM are paving the way with the FDA — through formal regulatory comments and informal dialogue — to enable mRNA to be considered a platform technology that will shorten development timelines. This collective effort will convert analytical insights into real-world benefits, reinforcing mRNA’s position as the fourth pillar of pharmaceutical innovation and intervention by delivering new mRNA-based medicines globally for patients.
References
- https://www.advancingrna.com/doc/mrna-the-fourth-pillar-of-pharmaceutical-innovation-and-intervention-0001
- Beacon RNA (Hanson Wade Group June 2024); https://beaconintelligence.com/solutions/rna/
- Analytical Procedures for mRNA Vaccine Quality. Draft Guidelines: 3rd Edition
- https://ir.arcturusrx.com/news-releases/news-release-details/japans-ministry-health-labour-and-welfare-approves-csl-and
- https://www.technologyreview.com/2024/02/02/1087536/the-next-generation-of-mrna-vaccines-is-on-its-way/
About the Authors:
 Khaled Yamout is the founder and CEO of Yamout Chem Consulting, LLC and is a thought leader in analytical sciences, quality, and manufacturing. He is a member of AMM and serves on multiple AMM committees, including the Science and Technology Leadership Committee. Also, he is a volunteer member with USP, where he serves as the chair of the USP Expert Panel on mRNA vaccines. Previously, he held a position as a senior director, analytical services and quality control at TriLink Biotechnologies, where he oversaw the Analytical Sciences Center of Excellence and all analytical aspects of method development and validation to product release and stability to support regulatory filings for both small and large molecules. Prior to TriLink, Yamout held various positions in quality control, R&D, and manufacturing, wherein he supported several drug substances and drug products (both small molecules and biologics) from clinical phase to commercial.
Khaled Yamout is the founder and CEO of Yamout Chem Consulting, LLC and is a thought leader in analytical sciences, quality, and manufacturing. He is a member of AMM and serves on multiple AMM committees, including the Science and Technology Leadership Committee. Also, he is a volunteer member with USP, where he serves as the chair of the USP Expert Panel on mRNA vaccines. Previously, he held a position as a senior director, analytical services and quality control at TriLink Biotechnologies, where he oversaw the Analytical Sciences Center of Excellence and all analytical aspects of method development and validation to product release and stability to support regulatory filings for both small and large molecules. Prior to TriLink, Yamout held various positions in quality control, R&D, and manufacturing, wherein he supported several drug substances and drug products (both small molecules and biologics) from clinical phase to commercial.
 Deborah (“Deb”) Day Barbara is a cofounder of the Alliance for mRNA Medicine (AMM) and the current chairperson for AMM’s sister organization, the Foundation for mRNA Medicine. A seasoned professional in the life sciences industry, she recently held the position of vice president of strategy & business development at Maravai LifeSciences Holdings, Inc. Prior to Maravai, she held leadership roles at ThermoFisher Scientific, Strategic Diagnostics, GeneLogic, the Johns Hopkins University, and Amersham Life Sciences. With over 30 years of experience, she has made significant contributions to the life sciences industry, particularly in the areas of strategic planning, business development, licensing, intellectual property management, and technology development. Barbara advocates for the advancement of mRNA medicines.
Deborah (“Deb”) Day Barbara is a cofounder of the Alliance for mRNA Medicine (AMM) and the current chairperson for AMM’s sister organization, the Foundation for mRNA Medicine. A seasoned professional in the life sciences industry, she recently held the position of vice president of strategy & business development at Maravai LifeSciences Holdings, Inc. Prior to Maravai, she held leadership roles at ThermoFisher Scientific, Strategic Diagnostics, GeneLogic, the Johns Hopkins University, and Amersham Life Sciences. With over 30 years of experience, she has made significant contributions to the life sciences industry, particularly in the areas of strategic planning, business development, licensing, intellectual property management, and technology development. Barbara advocates for the advancement of mRNA medicines.
