How To Optimize Downstream Separation With Tagless Proteins
By Milton Sabat Gonzalez-Serrano, Department of Chemical and Biomolecular Engineering, The Ohio State University
Antibody-based therapeutics dominate the market due to their efficient recombinant protein production and economic feasibility, benefiting from established purification systems like Protein A. These systems leverage the antibodies’ Fc molecular architecture, ensuring robust production and purification.
However, recombinant protein production for non-antibody biologics faces challenges, as they lack similar purification platforms. While recombinant affinity tags can improve protein expression, they often interfere with activity or binding, posing risks like immunogenicity in therapeutics, potentially excluding promising drug candidates from clinical trials.
Additionally, any therapeutic purification processes that rely on recombinant fusion tags require complex tag removal and separation steps to meet FDA safety guidelines. Proteases are often used to cleave tags but can create challenges in downstream processing, especially at larger scales. These processes also involve time-consuming design-of-experiment (DoE) protocols and multiple purification columns, increasing costs and delaying development. In some cases, proteases may also be chemically incompatible with the target protein, further complicating the process.
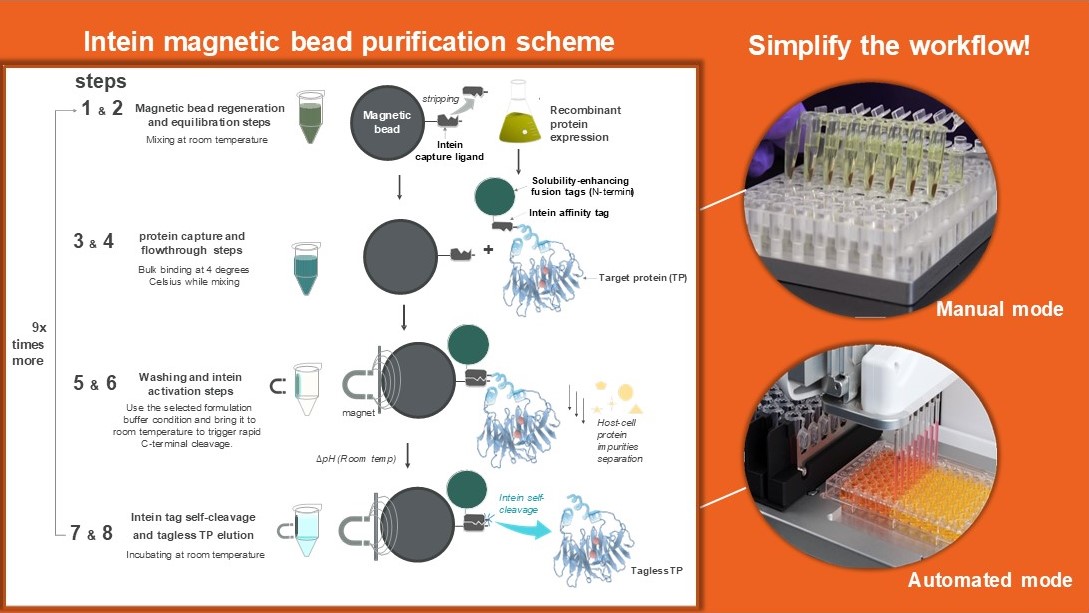
Recent innovations aim to address these challenges by engineering self-removing intein affinity tags to simplify and accelerate the development of non-antibody therapeutics. One such innovation is the engineered N. punctiforme split DnaE intein system, designed for enhanced C-terminal cleavage with pH controllability. In this system, one intein fragment is immobilized on a solid support as the capture ligand, while the other fragment is expressed as a fusion tag on the target protein’s N-terminus. The pH-controllable system allows impurity separation at an alkaline pH (8.5), suppressing cleavage, followed by a pH 6.2 wash that triggers rapid cleavage at room temperature, releasing the purified protein into the mobile phase.
While resin-based chromatography remains a primary means for protein purification, drug discovery increasingly requires faster, innovative protein isolation technologies. Combining self-cleaving inteins with magnetic bead support promises to accelerate biologic therapeutic development, from cell systems to analytical assays.
Magnetic Bead Bioseparation Presents A Novel Rapid Protein Purification Alternative
The biotechnology and biopharmaceutical industries are increasingly adopting integrated process development, with multidisciplinary teams focused on continuous analytical monitoring and biomolecular characterization. As the field advances, there is a noticeable shift toward developing therapeutics beyond traditional antibody-based treatments, including cytokines, single-chain variable fragments (scFvs), Fv-nanobody fusions, antigen binding fragments (Fab), bispecific T-cell engagers (BiTEs), enzymes, and other innovative protein biologics. This shift highlights the growing need for more efficient and cost-effective purification technologies to accelerate drug discovery, with high-throughput capabilities becoming essential for developing and commercializing new therapeutics.
A significant breakthrough in addressing this need is the integration of magnetic bead bioseparation platforms with self-cleaving inteins, resulting in a novel method for the rapid purification of challenging target proteins. Using inteins on a magnetic support provides several advantages by reducing downstream processing complexity, lowering costs, and accelerating the isolation of tagless therapeutics, all with a simple and efficient setup. The intein-based magnetic bead system enables effective affinity capture, tag removal, and buffer exchange, supporting direct analytical applications to meet the fast-paced demands of drug development.
These advancements are particularly relevant as clinical research shifts toward more personalized treatments. This innovative protein bioseparation platform provides an ergonomic and valuable solution for academic researchers, advancing protein science and expanding the possibilities within life sciences. Ongoing developments in this area continue to focus on enhancing bioprocesses and biotechnology, ensuring greater efficiency and accessibility for both therapeutic development and protein research.
Figure 1
Source: M. Sabat Gonzalez-Serrano
Intein-Based Beads Have Many Advantages, Including Long Useful Life
By leveraging the self-cleaving intein system, target proteins are efficiently released directly off the magnetic bead without recombinant tags, additional reagents, or enzymatic steps. This feature significantly accelerates the overall purification process, making it much more suitable for high-throughput screening of drug candidates, particularly when rapid results are critical, such as in drug discovery.
Magnetic bead platforms streamline bench-scale purifications by allowing beads to be easily mixed with the sample in a tube and rapidly separated using a magnetic tube rack. This significantly reduces processing time, as the mobile phase can be quickly decanted or pipetted out directly. In contrast, gravity chromatography columns require careful resin bed packing and additional time for the mobile phase to diffuse and flow through, making the magnetic bead approach a faster and more efficient alternative for protein purification. Furthermore, this technology can purify multiple therapeutic candidates in parallel, directly from clarified cell lysates or cell culture supernatant.
Processes can also be automated using a pipetting robot setup with magnets and a bespoke AI interface to automate workflows. This can free up time for the operator, and it can greatly improve the workflow for reliable capture and consolidation of multiple operation units such as tag removal, separation, and buffer exchange steps. Because the self-cleaving reaction can accommodate many buffer conditions, the TP elutes directly into the product formulation buffer or activity assay buffer without recombinant tags. This enables the bioanalytical team to immediately probe and characterize samples directly from elution for high-throughput data generation. Moreover, this purification platform proves to be an ergonomic alternative, as the intein-based magnetic bead was successfully regenerated and reused up to ten times, effectively extending the utility of such technology. The intein magnetic bead provides a competitive option to immunoprecipitation methods, which often rely on protein complex denaturants and refolding for effective recovery.
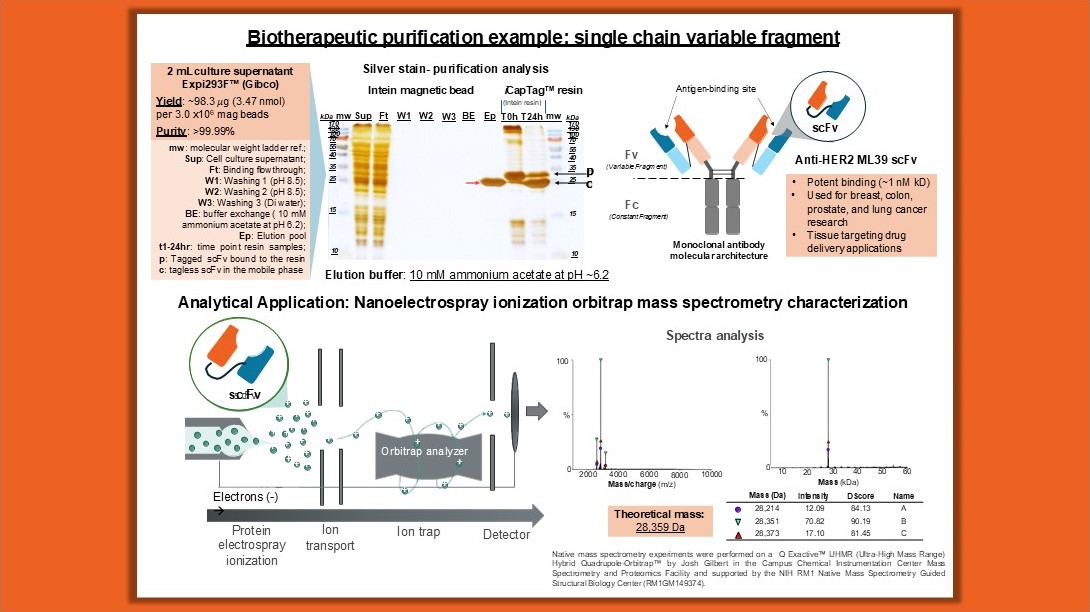
Our lab used this technology to generate tagless ML39 scFv, a ~28 kDa potent anti-HER2 chimeric single chain variable fragment for cancer research and other drug delivery applications. The intein-tagged scFv was successfully secreted into the culture media through selected signal peptide sequences as a strategy to simplify the sample processing and reduce the host-cell impurities. We used the developed intein magnetic beads to capture the scFvs directly from the culture supernatant. This method allowed the cleavage and release of scFvs without tags directly into mass spectrometry (MS)-compatible buffers for immediate nanospray ionization MS analysis, proving highly effective buffer exchange capabilities and analytical feasibility.
Another example is the human full-length wild-type bifunctional glutamyl-prolyl tRNA synthetase (FL-wtEPRS), a ~170 kDa cytosolic protein that attaches the correct amino acid onto the corresponding tRNA for protein synthesis. This molecule has drawn the attention of many researchers in the last few years, as recent studies have reported other non-canonical functions, with genetic mutation polymorphisms correlated to diabetes, cardiovascular disease, and other disorders. By combining large solubility fusion tags with a self-cleaving intein tag, the FL-wtEPRS was purified and released directly into the enzyme activity buffer without recombinant tags. This allowed for testing the aminoacylation activity of both domains of the bifunctional enzyme immediately after elution, demonstrating the power of this separation method for fast characterization.
Processes requiring stabilizing additives like detergents and metal ions can often limit protein purification, as many traditional affinity chromatography methods have narrow chemical tolerances. However, the intein-based magnetic bead system successfully purified the membrane-associated enzyme paraoxonase-1 (PON1), a ~39 kDa, a calcium-dependent enzyme with therapeutic potential in chemical defense and cardiovascular disease. In this case, PON1 was recombinantly produced in E. coli using solubility-enhancing fusion tags and a self-cleaving intein. Solubilization was achieved with tergitol detergent and calcium ions without affecting intein cleavage activity. Tagless PON1 was then eluted into the enzyme activity assay buffer, where its hydrolysis of phenol acetate substrate was tested. It was found that the catalytic efficiency of the tagless PON1 was comparable to the protease-generated tagless PON1 reported in the literature. Certainly, this purification example highlights the flexibility of this method and expands the possibilities to study other challenging therapeutics like receptors and membrane proteins.
Figure 2
Source: M. Sabat Gonzalez-Serrano
This versatile purification platform can be tailored to meet the specific needs of any target protein, allowing users to apply optimized conditions for continuous characterization and process analytical technology (PAT), advancing therapeutic development. Whether it is large or small therapeutics, the possibilities are endless. The intein magnetic bead prototype demonstrated in this work is currently being developed for commercialization by Protein Capture Science.
Obstacles Include Lengthy Validation Process
While integrating magnetic beads with self-cleaving intein tags shows significant promise for protein purification, several challenges could hinder its broader adoption. A key issue is the need for optimization when dealing with various therapeutic proteins, as self-cleavage efficiency can vary based on the first two amino acids in the fusion protein. Additionally, proteins with complex folding patterns or post-translational modifications may not cleave as efficiently, limiting the technology’s applicability for certain therapeutics. Another obstacle is the extensive validation and regulatory compliance required within the biopharmaceutical industry, which could slow its integration into existing production workflows.
Nevertheless, the intein purification system has successfully purified therapeutics with post-translational modifications such as erythropoietin, the SARS-CoV-2 receptor binding domain, interferon alpha b2, and other glycoproteins. The magnetic bead has already demonstrated ample versatility by successfully purifying membrane-associated targets like paraoxonase-1 using solubility-enhancing fusion tags and detergent solubilization additives.
Recent advances in intein protein engineering, including a new variant that accelerates cleavage for a broader range of target proteins, show great promise. This prototype also performs well at low temperatures while maintaining pH controllability, making it ideal for temperature-sensitive proteins. In addition, early development of ELISA validation assays reveals that this technology can produce elution with less than 100 per million (ppm) of tagged protein per dose of untagged TP, showing compliance with FDA biopharmaceutical safety guidelines. These advancements highlight the potential of this innovative high-throughput technology, not only for bench-scale research in academic institutions but also for drug discovery in the biopharmaceutical industry.
Conclusion
Integrating magnetic beads with self-cleaving intein tags marks a significant advancement in high-throughput protein separation, especially in developing therapeutic candidates. This innovative platform streamlines the purification process by eliminating the need for complex reagents, enhancing throughput, and improving efficiency. As a result, it offers a highly valuable tool for research and development. By addressing the limitations of traditional affinity tag-based purification systems, this technology reduces production costs, shortens timelines, and accelerates drug candidate screening with rapid and effective protein purification.
As the biopharmaceutical industry shifts toward more personalized therapies, this technology can potentially speed up the production of life-saving treatments, making them both more cost-effective and accessible. The flexibility of intein magnetic beads ensures more consistent and efficient production processes, particularly in high-throughput screening environments where time and efficiency are critical.
Ultimately, this innovation meets the growing demand for faster, simpler, and more cost-effective therapeutic development solutions tailored to each target’s specific needs. By streamlining processes, this technology can help accelerate drug discovery and advance the field of protein science.
About The Author:
 Milton Sabat Gonzalez-Serrano is completing his Ph.D. in biochemistry at The Ohio State University under Dr. David Wood in the Wood’s Applied Protein Engineering Laboratory. His research has focused on recombinant protein production, specifically therapeutic downstream processing and purification biotechnology. He received the NIH Molecular Biophysics Training Program (MBTP) fellowship, gaining expertise in molecular biophysics techniques. In 2022, he was awarded an international DAAD scholarship for a graduate internship abroad at the Institute for Bioprocess and Analytical Measurement Techniques e.V. (IBA) in Thüringen, Germany, and recently received a graduate school dissertation year fellowship.
Milton Sabat Gonzalez-Serrano is completing his Ph.D. in biochemistry at The Ohio State University under Dr. David Wood in the Wood’s Applied Protein Engineering Laboratory. His research has focused on recombinant protein production, specifically therapeutic downstream processing and purification biotechnology. He received the NIH Molecular Biophysics Training Program (MBTP) fellowship, gaining expertise in molecular biophysics techniques. In 2022, he was awarded an international DAAD scholarship for a graduate internship abroad at the Institute for Bioprocess and Analytical Measurement Techniques e.V. (IBA) in Thüringen, Germany, and recently received a graduate school dissertation year fellowship.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Outsourced Pharma? Subscribe today.