How To Implement Continuous Process Monitoring Of Validated Processes
By Mark Durivage, Quality Systems Compliance LLC
FDA regulations require “Where the results of a process cannot be fully verified by subsequent inspection and test, the process shall be validated with a high degree of assurance and approved according to established procedures.” However, validation is only half of this requirement. Validated processes must also be controlled and monitored, a requirement generally referred to as continuous process monitoring. Pharmaceutical, medical device, and human tissue regulations all require validation and continuous process monitoring of validated processes.
process shall be validated with a high degree of assurance and approved according to established procedures.” However, validation is only half of this requirement. Validated processes must also be controlled and monitored, a requirement generally referred to as continuous process monitoring. Pharmaceutical, medical device, and human tissue regulations all require validation and continuous process monitoring of validated processes.
Requirements
21 CFR Part 211 Current Good Manufacturing Practice for Finished Pharmaceuticals
Sec. 211.110 Sampling and testing of in-process materials and drug products.
(a) To assure batch uniformity and integrity of drug products, written procedures shall be established and followed that describe the in-process controls, and tests, or examinations to be conducted on appropriate samples of in-process materials of each batch. Such control procedures shall be established to monitor the output and to validate the performance of those manufacturing processes that may be responsible for causing variability in the characteristics of in-process material and the drug product.
(b) Valid in-process specifications for such characteristics shall be consistent with drug product final specifications and shall be derived from previous acceptable process average and process variability estimates where possible and determined by the application of suitable statistical procedures where appropriate. Examination and testing of samples shall assure that the drug product and in-process material conform to specifications.
(c) In-process materials shall be tested for identity, strength, quality, and purity as appropriate, and approved or rejected by the quality control unit, during the production process, e.g., at commencement or completion of significant phases or after storage for long periods.
21 CFR Part 820 Quality System Regulation
Sec. 820.70 Production and process controls.
(a) General. Each manufacturer shall develop, conduct, control, and monitor production processes to ensure that a device conforms to its specifications. Where deviations from device specifications could occur as a result of the manufacturing process, the manufacturer shall establish and maintain process control procedures that describe any process controls necessary to ensure conformance to specifications. Where process controls are needed they shall include:
(1) Documented instructions, standard operating procedures (SOP's), and methods that define and control the manner of production;
(2) Monitoring and control of process parameters and component and device characteristics during production;
(3) Compliance with specified reference standards or codes;
(4) The approval of processes and process equipment; and
(5) Criteria for workmanship which shall be expressed in documented standards or by means of identified and approved representative samples.
21 CFR Part 1271 Human Cells, Tissues, And Cellular and Tissue-Based Products
Sec. 1271.220 Processing and process controls.
(a) General. If you are an establishment that processes HCT/Ps, you must process each HCT/P in a way that does not cause contamination or cross-contamination during processing, and that prevents the introduction, transmission, or spread of communicable disease through the use of the HCT/P.
(c) In-process control and testing. You must ensure that specified requirements, consistent with paragraph (a) of this section, for in-process controls are met, and that each in-process HCT/P is controlled until the required inspection and tests or other verification activities have been completed, or necessary approvals are received and documented. Sampling of in-process HCT/Ps must be representative of the material to be evaluated.
Regulatory Actions
During 2019, the FDA issued 168 inspectional 483 observations specifically citing inadequate or the lack of continuous process monitoring. The following table is derived from FDA 483 observations issued between Oct. 1, 2018 and Sept. 30, 2019.
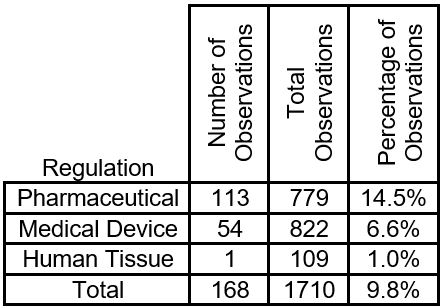
As shown in the table, overall continuous process monitoring accounts for 9.8% of the 483 observations issued by the FDA.
Among some of the comments relating to the citations are:
- Your examination and testing of samples did not assure that the drug product and in-process material conformed to specifications.
- Your in-process specifications for sampling and testing of in-process materials and drug products [were inconsistent with drug product final specifications] [were not derived from previous acceptable process average and process variability estimates] [were not determined by the application of suitable statistical procedures].
- Process control procedures that describe any process controls necessary to ensure conformance to specifications have not been [adequately] established.
- Production processes were not [developed] [conducted] [controlled] [monitored] to ensure that a device conforms to its specifications.
- HCT/Ps were not processed in a way [that does not cause contamination or cross contamination during processing] [that does not increase the risk of introduction, transmission, or spread of communicable disease].
Continuous Process Monitoring
Once a process is validated, a plan for continuous process monitoring should be established proportionate to the risk involved with the product or process. The first step is to ensure the process parameters are set and maintained within the ranges established during the validation.
The most useful tools that I utilize for continuous process monitoring include acceptance sampling, periodic inspections, and control charts. Of these techniques, acceptance sampling is the least preferred, as the sampling is not performed in real time, which does not allow for process adjustments.
Periodic inspections are preferable to acceptance sampling. Periodic inspections may allow for some process adjustment, but the biggest advantage over acceptance sampling is the ability to bracket products to the last known good parts, thereby minimizing the risk of discrepant products being further processed or distributed. Supplementing the periodic inspection method is the practice of first and last piece inspection. First and last piece inspection is also an effective method to bracket discrepant products.
Control charts build upon periodic inspections by plotting the process outputs and monitoring the process for special cause variation or trends. Control charts are decision-making tools that provide information for timely decisions concerning recently produced products. Control charts contain a centerline — usually the mathematical average of the samples plotted — and upper and lower statistical control limits that define the constraints of common cause variation and performance data plotted over time.
There are two general classifications of control charts: variables and attributes charts. Variables are things that can be measured. Attributes are things that can be counted. The type of data (variable or attribute) will dictate the appropriate type of control chart required to monitor a process. The following table can be used for control chart selection.
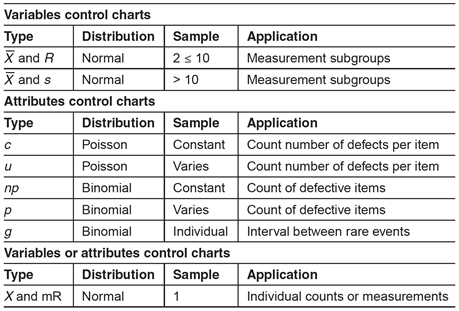
Selection of the correct type of control chart is important to ensure the underlying statistical concepts are appropriate for the feature or attribute being measured.
A process is said to be in control when the control chart does not indicate any out-of-control condition and contains only common causes of variation. If the common cause variation is small, then a control chart can be used to monitor the process. If the common cause variation is too large, the process will need to be modified.
When a control chart indicates an out-of-control condition (a point outside the control limits or matching one or more of the criteria in the rules below), the assignable causes of variation must be identified and eliminated.
The following rules can be used to properly interpret control charts:
Rule 1 - One point beyond the 3 σ control limit
Rule 2 - Eight or more points on one side of the centerline without crossing
Rule 3 - Four out of five points in zone B or beyond
Rule 4 - Six points or more in a row steadily increasing or decreasing
Rule 5 - Two out of three points in zone A
Rule 6 - 14 points in a row alternating up and down
Rule 7 - Any noticeable/predictable pattern, cycle, or trend
Conclusion
It is time to consider augmenting your validated pharmaceutical, medical device, and tissue production processes, including processing, packaging, and labeling, with continuous process monitoring to ensure continued compliance with established specifications and requirements if you do not want to be one of the 9.8% cited by the FDA.
I cannot emphasize enough the importance of establishing documented procedures to manage the tools and methods used. Best practice includes providing rationales for your organization’s use of continuous process monitoring tools and activities. The requirements and continuous process monitoring tools presented in this article can and should be utilized based upon industry practice, guidance documents, and regulatory requirements.
References:
- Durivage, M.A., 2014, Practical Engineering, Process, and Reliability Statistics, Milwaukee, ASQ Quality Press
- Durivage, M.A., and Mehta, B., 2016, Practical Process Validation, Milwaukee, ASQ
Quality Press
- https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?CFRPart=211
- https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?CFRPart=820
- https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?CFRPart=1271
- https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-references/inspection-observations
About The Author:
 Mark Allen Durivage has worked as a practitioner, educator, consultant, and author. He is managing principal consultant at Quality Systems Compliance LLC, an ASQ Fellow, and an SRE Fellow. Durivage primarily works with companies in FDA-regulated industries (medical devices, human tissue, animal tissue, and pharmaceuticals), focusing on quality management system implementation, integration, updates, and training. Additionally, he assists companies by providing internal and external audit support as well as FDA 483 and warning letter response and remediation services. He earned a BAS in computer aided machining from Siena Heights University and an MS in quality management from Eastern Michigan University. He holds several certifications including CRE, CQE, CQA, CSSBB, RAC (Global), and CTBS. He has written several books available through ASQ Quality Press, published articles in Quality Progress, and is a frequent contributor to Life Science Connect. You can reach him at mark.durivage@qscompliance.com and connect with him on LinkedIn.
Mark Allen Durivage has worked as a practitioner, educator, consultant, and author. He is managing principal consultant at Quality Systems Compliance LLC, an ASQ Fellow, and an SRE Fellow. Durivage primarily works with companies in FDA-regulated industries (medical devices, human tissue, animal tissue, and pharmaceuticals), focusing on quality management system implementation, integration, updates, and training. Additionally, he assists companies by providing internal and external audit support as well as FDA 483 and warning letter response and remediation services. He earned a BAS in computer aided machining from Siena Heights University and an MS in quality management from Eastern Michigan University. He holds several certifications including CRE, CQE, CQA, CSSBB, RAC (Global), and CTBS. He has written several books available through ASQ Quality Press, published articles in Quality Progress, and is a frequent contributor to Life Science Connect. You can reach him at mark.durivage@qscompliance.com and connect with him on LinkedIn.
