How To Ace Your Next CDMO Capabilities Audit
By Ray Sison

Site visits require a significant investment of resources on the part of both a sponsor and its prospective CDMO, so swapping out the time required to prep, travel, meet, and follow up in favor of a two-hour teleconference is understandable … to a limited extent. There are times when avoiding face-to-face meetings would simply be negligent — and the tradeoff unjustifiable — and those times include CDMO selection due diligence, quality audits, and critical operations.

Why Conduct An On-Site Capabilities Audit?
A capabilities audit is an integral part of a sponsor's due diligence in vendor procurement. It is often the sponsor’s first exposure to a CDMO’s operations, and the primary objectives are to evaluate the facility, equipment, quality systems, and the expertise of each functional team in relation to the specific needs of the project.
During a competitive bid selection process, solicit multiple (three to five) proposals and use the CDMOs’ overall quality to preliminarily rank the field. Factor in predetermined criteria to identify no more than two or three companies with whom to schedule visits. This will balance resource conservation with adequate data collection to enable objective comparisons. As a side benefit, the process will test your assumptions and provide new perspectives on the challenges that may lie ahead.
Be clear with the CDMOs about your goals for the visits, and communicate those goals to your internal stakeholders, as well.
Who Should Attend The Audit(s)?
Assemble a cross-functional travel team in order to formulate a comprehensive evaluation of the CDMOs. Depending on the stage of the project (e.g., development vs. commercial tech transfer), the team may consist of internal stakeholders from:
- R&D
- Technical services
- Quality assurance
- Regulatory
- Procurement
At clinical- or development-stage companies, roles and functions may not be formally defined. But even if resources are limited, the travel team should include either technical and quality functions or at least two project stakeholders. Note that while there is some overlap between capabilities and quality audits, they are distinct activities. If timing or other circumstances require these two types of site visits to be conducted in parallel, understand that a CDMO will view each audit as separate events, each with its own agenda and personnel requirements for attendance. Ideally, these audits should be addressed in sequence: two to three capabilities audits followed by only one to two quality audits, within a reasonable timeframe.
Preparing For The Capabilities Audit
Conducting a site visit is not a passive exercise. Set the tone by sending a suggested agenda with a requested audit date. Because a business development rep is your primary contact at this stage, other CDMO site personnel may not be as familiar with your company, your project, or your people coming on site. The agenda and a brief project summary will help frame the talks and make time spent at the facility more efficient. Identify the names and titles of your travel team in the agenda. You can also ask if the CDMO has a standard agenda for site visits and allow for adjustments to your agenda that make sense. To save time, ask the CDMO if they can provide an information sheet about the facility and suggest travel arrangements, including local area hotels and restaurants.

Figure 1: Sample agenda
I discourage presenting lengthy corporate slide decks or data-intensive research expositions during the visit. Each side’s presentation should provide appropriate background and context for the visit. Prepare no more than three to four slides, and circulate them prior to the meeting. CDMOs often feel disconnected from the patients they will ultimately impact — you can close this gap by including a high-level clinical overview of the disease and information about product requirements in your presentation. If the drug product has innovative attributes, bring samples to show the CDMO’s team.
Since visits should not occur before a set of comparable proposals are received, review the proposals and compile questions and comments from your team, and then submit a clean set to the CDMO prior to the visit. This will give them time to prepare responses and will allow for a more productive meeting. Time permitting, review the master services agreement (MSA) template or general terms and conditions for any glaring conflicts. Since the proposal represents the CDMO's understanding of the project and their customized response to a well-crafted request for proposal (RFP), it will be the basis for that day’s technical discussion. Walking through the proposal with them should reveal their depth of experience, ideas they can bring to the table, and applicable expertise they have to your specific project. Find ways to prime that discussion.
Additionally, review the selection criteria as a team prior to the audit, and then decide if the priorities and weightings are still valid — or need to be updated based on new information and/or changing circumstances. Scan public information for company announcements from the CDMO or news or events (e.g., FDA audits) that need to be addressed. It is not unheard of for a CDMO to radically change direction, get acquired, or even be shut down by the FDA during a selection process.
Conducting The Site Visit
On the day of the site visit, be sure to have all supporting information organized and confer with your team about the meeting objectives and discussion points. Walk through the agenda together and assign a facilitator and/or leader for each section. Try to put all other projects and distractions on hold to focus solely on the task at hand. Be present and engaged, and expect no less from the vendor.
Despite the improvements in video conferencing technology, there is still no substitute for personal introductions and first impressions in building long-term relationships. Pay attention to nonverbal cues — body language and tone can convey a trove of useful information, for example when a CDMO representative is describing their response to an FDA 483 observation. A brief conversation with a mechanic or engineer setting up and operating equipment can trigger an informative technical discussion and reveal novel approaches to process development and more. In short, wring as much value from the experience on site as time will allow.
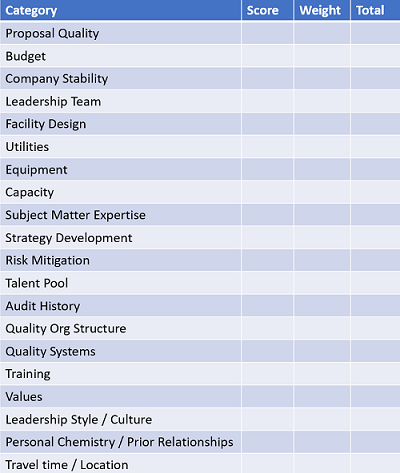
Afterwards, don’t wait to compile objective measures, e.g., selection criteria scoring. Debrief with colleagues in the parking lot or at the airport, while impressions are still fresh. Personally, I favor a methodological approach (see Table 1) over “blink” impressions, but don’t deny the importance of gut feel. However, subjective impressions should only be weighed after objective measures are evaluated.
Table 1: Sample Selection Criteria Worksheet
10 Site Audit Best Practices
In summary, here are some tips to help you get the most value out of your site visit:
- Control the process. Set the agenda by sending them an agenda, questions, and discussion points ahead of the meeting.
- Don't be sold. Keep your priorities straight. Use the selection criteria worksheet as a tool to foster discussions and stay on track.
- Think 360 … and then some. Consider cross-functional perspectives, and be open to all the tangible and intangible forms of communication. Be aware that they will be reading you, as well.
- Challenge your assumptions. Listen carefully to their questions, concerns, and strategies. React to information and observations, and see if they match previous impressions.
- Don't rely on memory. Complete scoring of selection criteria and consolidate notes with your team immediately after each site visit, while impressions are fresh.
- Be objective. Let the site visit strengthen the conviction of your ranking or realign your expectations.
- Conserve time, money, and energy. Ask for travel tips to get there and back, and create templates where needed.
- Respect everyone's time. Be on time, allot enough time, and stay on track.
- Get inside their heads and hearts. The site visit will be the best early indicator of how they think and what values guide an organization.
- Allow room for subjectivity. After all objective measures are addressed, let the team weigh in about their gut feelings regarding the site visit, personal chemistry, confidence of success, etc.
Whether you are a road warrior, an overextended multitasker tethered to a desk, or a little of both, on-site capability assessments are an indispensable part of the CDMO selection process. Be prepared, stay focused, and follow through immediately for an efficient and effective site visit.
About The Author
 Ray Sison is VP of pharmaceutical outsourcing and tech transfer at xCell Strategic Consulting. He began consulting in 2011 after recognizing a need for expertise in pharmaceutical outsourcing among the discovery- and clinical-stage pharma companies he served as a business development representative for Patheon and MDS Pharma Services. Based on his experience, Sison provides insight to the CDMO’s business and operations, helping his clients negotiate and achieve better outcomes. Additionally, he has developed sound processes and templates to streamline CMO procurement to save time and cost. In this series of articles, as well as online webinars, he continues to share best practices and case studies, helping improve the outsourced business model. You can reach him at rsison@xcellstrategicconsulting.com or connect with him on LinkedIn.
Ray Sison is VP of pharmaceutical outsourcing and tech transfer at xCell Strategic Consulting. He began consulting in 2011 after recognizing a need for expertise in pharmaceutical outsourcing among the discovery- and clinical-stage pharma companies he served as a business development representative for Patheon and MDS Pharma Services. Based on his experience, Sison provides insight to the CDMO’s business and operations, helping his clients negotiate and achieve better outcomes. Additionally, he has developed sound processes and templates to streamline CMO procurement to save time and cost. In this series of articles, as well as online webinars, he continues to share best practices and case studies, helping improve the outsourced business model. You can reach him at rsison@xcellstrategicconsulting.com or connect with him on LinkedIn.
