Essential Steps For A Successful Analytical Tech Transfer

Every tech transfer is unique, especially for complex biologics that aren’t well characterized yet, but at the risk of oversimplifying, each one shares commonalities.
Understanding the phases and key components of a successful tech transfer is increasingly important as more biotech companies depend on CDMOs for production of their clinical and commercial supply. Specifically, the reliance on mammalian cell culture platforms for biologics manufacturing at CDMOs has risen from 42.4% in 2006 to 70.3% in 2023, according to BioPlan Associates’ Report and Survey of Biopharmaceutical Manufacturing Capacity and Production.
Over the past two decades, the biotech industry has undergone significant transformation, shifting toward more advanced therapeutic solutions. Around 90% of biotech firms now collaborate with CDMOs to navigate the clinical trial process and bring their therapies to market. This trend highlights the increasing dependence on CDMOs for biologics manufacturing. Furthermore, CDMOs are becoming essential for smaller biopharmaceutical companies that lack the infrastructure and expertise to scale production for clinical trials or commercial distribution. Notably, between 2018 and 2023, 40% of biologics were launched by companies with limited or no prior commercialization experience, according to a 2023 analysis of FDA drug approvals.
CDMOs provide a wide range of services, including process development, production for toxicology and clinical studies, and fill/finish operations, as well as quality assurance (QA) and quality control (QC). To ensure the safety and efficacy of products intended for patient use, the FDA has established regulatory guidelines under the Code of Federal Regulations - Title 21 - Food and Drugs | FDA. Additionally, the International Council for Harmonization (ICH) has developed the ICH Q2(R2) guideline, which outlines detailed recommendations for validating analytical procedures in pharmaceutical development and manufacturing. This guidance applies to both drug substances and drug products, ensuring that analytical methods are robust, reliable, and fit for their intended purpose.
The transfer of processes or technologies at CDMOs is often a complex and time-intensive task. Specifically, the transfer of analytical methods can take up to 18 months to complete. This process involves ensuring that analytical methods are properly validated and can be reproduced reliably at the receiving site. For complex biologics, such as adeno-associated viruses (AAVs), the challenge is even greater. These products typically require the transfer of 15 to 20 analytical methods, depending on the specifics of the manufacturing process. These methods are critical to ensuring the drug product meets the necessary quality standards for release.
Any analytical method transferred to a CDMO or external testing site follows a path like this one:
- Preparation Phase (three months):
- Development of a detailed protocol outlining the method transfer process.
- Compilation of key documents, such as validation summaries and procedural instructions.
- Training and equipping personnel at the receiving facility for successful implementation.
- Execution Phase (six to eight months):
- Performing comparative evaluations or collaborative validation exercises between the sending and receiving laboratories.
- Testing established control samples to verify reliability and uniformity.
- Recording observations and conducting an in-depth analysis of results.
- Organizing the gathered data and preparing a detailed report on the method transfer process.
- Addressing any discrepancies or deviations identified during the transfer.
- Issuing final approval for the transfer results.
- Evaluation and Reporting (three months):
- Consolidating the collected data and preparing a comprehensive report outlining the details of the method transfer.
- Addressing and rectifying any identified deviations or inconsistencies during the process.
- Granting final approval to confirm the successful completion of the method transfer.
- Post-Transfer Activities:
- Incorporating the transferred method into routine testing workflows at the receiving laboratory.
- Monitoring the method's performance and addressing any issues that arise during implementation.
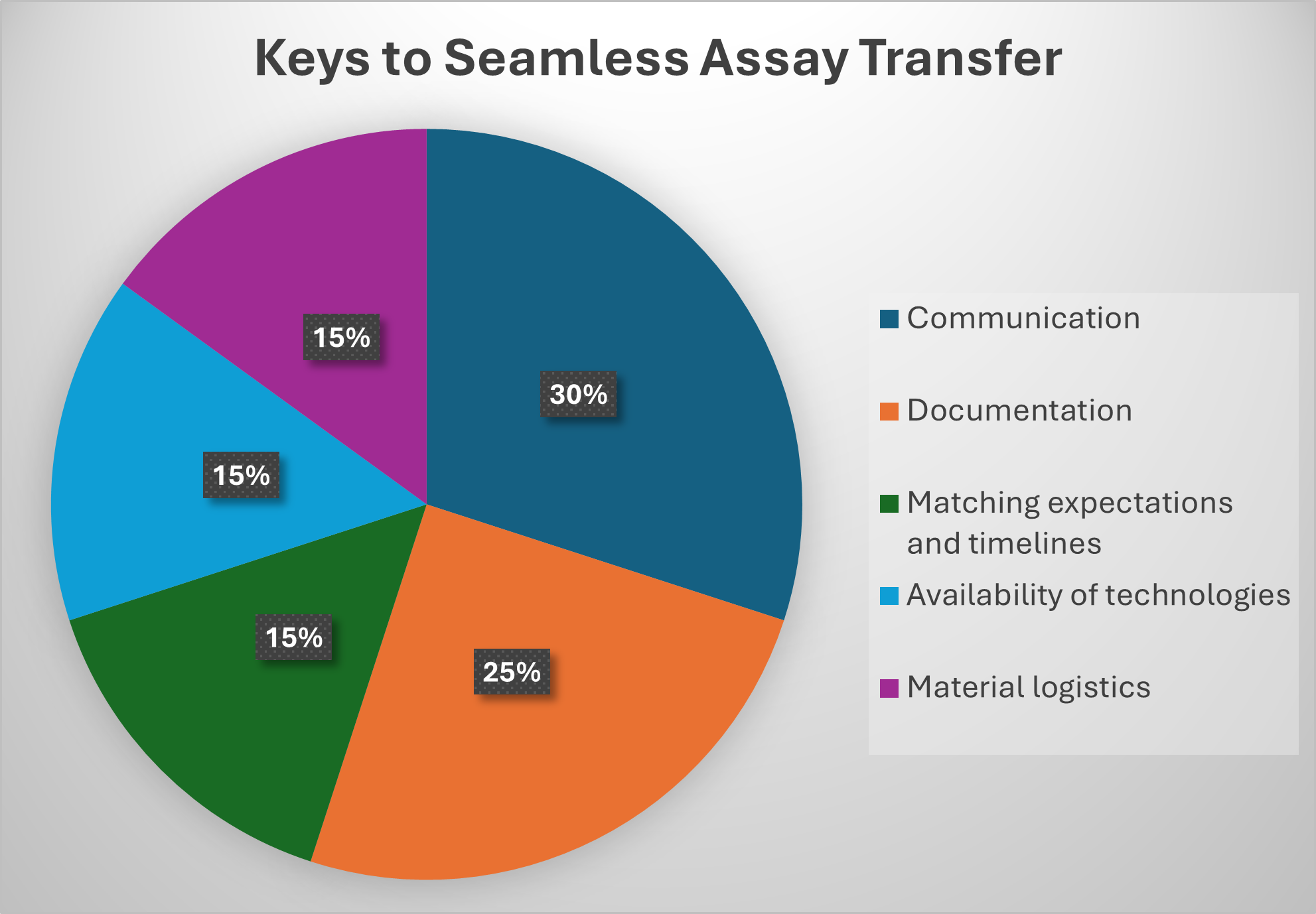
Essential components that ensure a seamless analytical assay transfer process:
- Communication: Effective communication plays a pivotal role in technology transfer, facilitating the smooth handover of technologies, processes, or knowledge from one group (such as research and development teams) to another (like manufacturing teams or external collaborators). Clear and efficient communication ensures the technology is implemented, scaled, and utilized effectively, minimizing disruptions and avoiding misunderstandings.
Tips for ensuring better communication:
- Plan regular meetings: Arrange consistent communication sessions between the transferring and receiving teams to provide updates, clarify details, and address any challenges encountered.
- Promote knowledge sharing: Conduct hands-on demonstrations to help the receiving team fully grasp the technology, and involve subject matter experts in discussions.
- Establish clear roles and responsibilities: Define who is accountable for each step of the transfer process, and assign liaisons or coordinators to streamline communication between teams.
- Implement a feedback system: Develop a process to gather and address concerns or suggestions from the receiving team, using this input to refine processes and enhance future transfers.
- Utilize collaborative tools: Make use of project management and communication platforms like Slack, Microsoft Teams, or Trello to monitor progress and share updates. Maintain shared repositories for documents and resources.
- Documentation: Accurate documentation plays a critical role in effectively conveying the knowledge, processes, and intellectual property tied to a technology, ensuring its successful implementation. In technology transfer, documentation can take various forms, such as knowledge sharing through standard operating procedures (SOPs), method standardization, and meeting regulatory compliance requirements.
Tips for ensuring better documentation:
- Record every detail of events and experiments: Thoroughly document every event, experiment, or observation to maintain traceability, ensure compliance, and facilitate continuous improvement. Adhere to ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, and Complete) to uphold data integrity and good documentation practices (GDP).
- Maintain clarity and accuracy: Ensure documentation is clear and precise to avoid misunderstandings, reduce errors, and comply with standards. Use standardized templates and simplify complex sentences to enhance readability and consistency.
- Secure approvals from key personnel: Obtaining approval for documentation is essential to guarantee accuracy, regulatory compliance, and operational effectiveness. Involving relevant stakeholders in the approval process ensures alignment with regulatory requirements and organizational objectives.
- Matching expectations and timelines: Ensuring alignment of expectations and timelines between the transferring and receiving teams is vital for a smooth process. Discrepancies in expectations or overly ambitious timelines can result in delays, inefficiencies, and tension between the parties involved.
Tips for ensuring matching expectations and timelines:
- Define clear objectives: Clearly articulate the purpose and scope of the technology transfer, including any potential challenges, limitations, or risks that might affect timelines or deliverables.
- Develop a detailed transfer plan: For biologics, particularly AAVs, ensure the analytical package is comprehensive. Conduct risk assessments during method transfers to prioritize tasks based on their complexity and the severity of the assay. Engage all stakeholders, including senior management at the recipient site, in the planning process.
- Involve stakeholders early: Early engagement of stakeholders is essential to address potential challenges, align objectives, and ensure smooth execution. This approach allows stakeholders to contribute their expertise, identify risks, and collaboratively create solutions.
- Manage expectations realistically: Be transparent about challenges, limitations, or risks that could influence timelines or outcomes. Ensure that quality and compliance are not sacrificed to meet tight deadlines.
- Embrace flexibility: Analytical technology transfers can be complex and time-consuming, requiring patience and resilience for successful outcomes.
- Availability of technologies: CDMOs have undergone significant advancements, increasingly adopting specialized technologies to facilitate the production of complex drug modalities. These innovations enable CDMOs to address the growing demand for personalized medicines, biologics, and advanced therapies, ensuring efficiency and scalability in the manufacturing process.
Tips for selecting the right CDMOs:
- Ensure assays are simple and straightforward: Avoid assays that require complex or sophisticated instrumentation, as integrating new technology at a CDMO can take several months to a year to prepare the equipment for operational use. This is a significant challenge and often a key factor in slowing down the transfer process.
- Select CDMOs carefully: With hundreds of CDMOs specializing in gene therapy, finding one that meets your requirements can be difficult. Conduct site visits to evaluate their capabilities, understand their offerings, and design your assays based on validated technologies and readily available instruments.
- Material logistics: Material is highly valuable, and the quantity needed for analytical transfers depends on various factors, such as the type of analysis, the number of tests required, and the specific methods being validated or transferred. It is crucial to ensure an adequate supply of material to support the entire transfer process and to use the same material as a control for the assays.
Tips for smooth material logistics for the entire transfer:
- Estimate the material needed: The required quantity of material varies by assay. For example, microbial/sterility and potency assays may require a few milliliters per run, while HPLC or qPCR assays might need only a few milliliters for the entire process. Calculate the material needed for each assay transfer and plan for an additional 30% to account for repeats or troubleshooting.
- Track inventory closely: Given material limitations, it is essential to maintain an up-to-date inventory. Many tools on the market offer inventory management services. Implementing a stringent workflow is highly recommended to maintain consistency and prevent inventory depletion. Avoid waiting until the last vial is used, as introducing new material will necessitate bridging experiments, which can consume a significant amount of material.
Fig. 1: Sample breakdown of the elements of an assay transfer
These are the author's viewpoints. They are not associated with his current employer.
References:
- Biopharma Mfg Capacity and Production 2024 - BioPlan Associates
- The Pharmaceutical Industry in 2023: An Analysis of FDA Drug Approvals from the Perspective of Molecules - PMC
About The Author:
 Rishi Ramesh Kothari is a process development engineer at Regeneron’s Pre-Clinical Manufacturing and Process Development team focusing on development of analytical assays and achieving process development goals for adeno-associated viruses going into clinic. He received his master’s degree in biotechnology from Drexel University College of Medicine.
Rishi Ramesh Kothari is a process development engineer at Regeneron’s Pre-Clinical Manufacturing and Process Development team focusing on development of analytical assays and achieving process development goals for adeno-associated viruses going into clinic. He received his master’s degree in biotechnology from Drexel University College of Medicine.
