Equipment You Need At Your Cell-And-Gene CDMO

By Louis Garguilo, Chief Editor, Outsourced Pharma

What equipment is essential at your cell and gene support facility?
I’ve received some answers via our look into Harvard University’s recently announced Center for Advanced Biological Innovation and Manufacturing, and a discussion with Emmanuel Ligner, CEO & President, GE Healthcare Life Sciences.
A Patient Cycle
Lets start with this diagram on the development and “manufacturing” of a gene-modified cell therapy. (More on those quotation marks surrounding manufacturing later.)
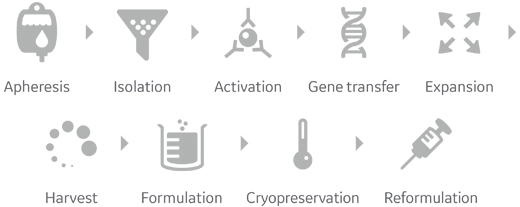
Note this process actually starts and ends with the patient. The workflow here is for autologous gene-modified cell therapy (utilizing the specific patient’s cells), and would vary for ex-vivo gene therapy. (We’ll get back to this below as well.)
GE’s Ligner begins our equipment edification with a phrase he says has driven the industry, and the need for these new processes and equipment: personalized medicine.

“Thus we should consider the first manufacturing-process-related activity at the facility as safe sample reception,” says Ligner.
“Next are processes for enriching the therapeutically valuable cells within the blood. Historically, the basic equipment for this has been a centrifuge. Considering ‘production equipment,’ a centrifuge of some type really is the first piece.”
Centrifuges, of course, vary in size and capabilities, and come with some drawbacks, including labor intensity, careful balancing of loads, and the fact they have been “open” systems, albeit often operated in a cleanroom environment.
GE among others had anticipated the increased, shall we say, centricity of the centrifuge. Some years ago, it purchased BioSafe, a specialized, closed-centrifuge producer.
“This can now be described as a fully automated centrifuge, incorporating control and optical sensors to perform ‘walk-away cell processing’ using a variety of customized protocols,” explains Ligner.
Having then isolated the white blood cells, they are “activated for expansion and subjected to a viral vector to deliver their genetic payload.”
Production of that viral vector, in turn, requires a separate “vector manufacturing suite.”
Viral vectors can be thought of as “tools” – most commonly various forms of virus employed to deliver (new/altered/repaired) genes into patients’ cells.
For example, pathogenic genes in a virus are commonly removed and altered for safety reasons, and to make room for the addition of “correct” genes to be delivered and expressed in target cells. When the virus "infects" the target cell, the gene of interest is expressed.
“The transgenic modification, or “transduction” of, for example, CAR-T cells, can be done in the centrifuge or can be done within a bioreactor itself. In the simplest terms, it involves exposing the cells of interest to the manufactured virus.
The next step is to have the genetically modified white blood cells multiply – often to much larger volumes. (As we learned in part one this step is often a less-than-robust process, and in need of some breakthrough improvements.)
For these required scale ups, bioreactors – which Ligner deems “auxiliary bioreactors” – are employed.
These can be single-use reactors, and vary from bench top (less than 1 liter to about 5 liter typically) to large scale, single-use or stainless steel equipment (50 liter to 2000 liter is not uncommon).
The specific selection of bioreactor should be driven by cell type and scale of production, “and a multipurpose facility will require several different bioreactor sizes and production configurations.”
Once the adequate volume and required concentration (density of cells) is obtained, says Ligner, “you are ready for down-stream processing [washing and concentrating cells], and fill and finish preparation for final infusion back into the patients.”
“This is accomplished using various equipment – typically a closed centrifuge again – capable of handling the volumes coming from the bioreactor, and affording additional liquid management to keep the system closed throughout the process.”
Vital to the (even short-term) storing and transport of the therapy is what Ligner refers to as “cryo protection.”
Equipment to carefully freeze cells under controlled conditions to maintain their viability and potency, for example, and then cold store and transport, is essential. (Anticipating this growing need, GE acquired Asymptote, a maker of “cryochain technology.”)
At the final end of the production cycle, Ligner reminds me, it is equally essential for the accurate and careful thawing of the product before injection.
“The product must be warmed up again, according to very controlled protocols, so you don't damage your cells,” he explains.
“The cool down to a freezing point and warm up to an infusion point are done with two different pieces of equipment. You must have appropriate thawing equipment at the point of care of the patient. The thawing equipment should be capable of working with a variety of different product configurations, from vials to product bags specially designed for infusion procedures.”
“Manufacturing” Vital Viral Vectors
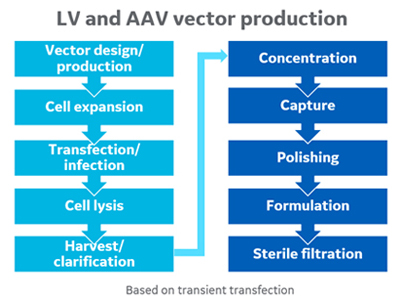
In this second diagram, “LV” is lentiviral vector, and “AAV” is adeno-associated virus. As we have mentioned, the production of viral vectors in sufficient quantities for clinical applications remains a high hurdle. Ligner reiterates that to genetically modify a cell, you need a viral vector, and for viral vector manufacturing there is a “large list of equipment that can be utilized.”
“If you think about cell therapy, you modify genetically the cell of the patient ex vivo using a viral vector. If you think about gene therapy, that modification occurs directly in the patient.
“But in both cases, you need viral vectors to carry the gene inside the cells, either ex vivo or in vivo.”
Furthermore, and the reason I enclosed “manufacture” in quotations above, the production of viral vectors takes on quite a different meaning from, for example, small-molecule API manufacture.
“Think about it this way,” says Ligner, selecting a monoclonal antibody (mAb) as an example. “Your ‘factory’ for the antibody is actually your cell – perhaps a Chinese hamster ovary (CHO) or other mammalian cell.
“So while we have all this supporting equipment, in essence the cell is your factory. You need to modify this and grow it in very large quantities. And then the cell is going to express protein, which you purify”
A distinction to note:
Autologous CAR T-cell therapies are created by genetically modifying a patient's own immune cells to target specific cancer cells before injecting them back into the patient.
Conversely, in an allogeneic CAR T-cell therapy, immune cells are instead collected from healthy donors (rather than specific patients), and genetically modified to make them “compatible with patients in an off-the-shelf manner.”
Concludes Ligner regarding both the personalized and the allogeneic approaches: “We have equipment for that.”
Make sure your own facility or your CDMO’s does, too.
