Environmental Isolates: What's The Proper Use Of In-House Cultures?
By Crystal M. Booth, PSC Biotech
 Use of environmental isolates in the pharmaceutical microbiology laboratory has been increasing in popularity, and, in some instances, warning letters have been written for not using environmental isolates in microbiological testing. However, because the available regulations and guidance documents are not clear regarding the use of environmental isolates, opinions vary on how to use them appropriately. This article discusses the current guidance documents, regulatory expectations, and expert opinions on the use of environmental isolates.
Use of environmental isolates in the pharmaceutical microbiology laboratory has been increasing in popularity, and, in some instances, warning letters have been written for not using environmental isolates in microbiological testing. However, because the available regulations and guidance documents are not clear regarding the use of environmental isolates, opinions vary on how to use them appropriately. This article discusses the current guidance documents, regulatory expectations, and expert opinions on the use of environmental isolates.
Overview
The term environmental isolate is understood to refer to cultures of microorganisms that have been recovered from the environment. This article will use the general term EIs to encompass microorganisms recovered from the environment, utility systems, or microbial assays.
Environmental isolates may be maintained in-house by the laboratory or sent to contract companies that can transfer the isolates into a ready-to-use format, such as <100 colony forming units (CFUs) per 0.1 milliliter (mL). The isolates can be cultured and maintained for up to five passages from the original colony for laboratory use. The requirement for isolates to be not more than five passages removed from the original master seed lot is mentioned in several United States Pharmacopeia (USP) chapters, including USP <61>, USP <62>, USP <71>, and USP <51>.1, 2, 3, 4 The purpose behind this requirement is to minimize phenotypic changes that may occur in the microorganisms with repeated culturing.5
But why is the use of EIs gaining momentum in microbial assays when the compendial documents don’t require the inclusion of EIs? Current industry opinions vary, and guidance documents are vague on the topic. The table below describes some excerpts from available guidance documents and whether the use of environmental isolates is required, not required, or recommended.
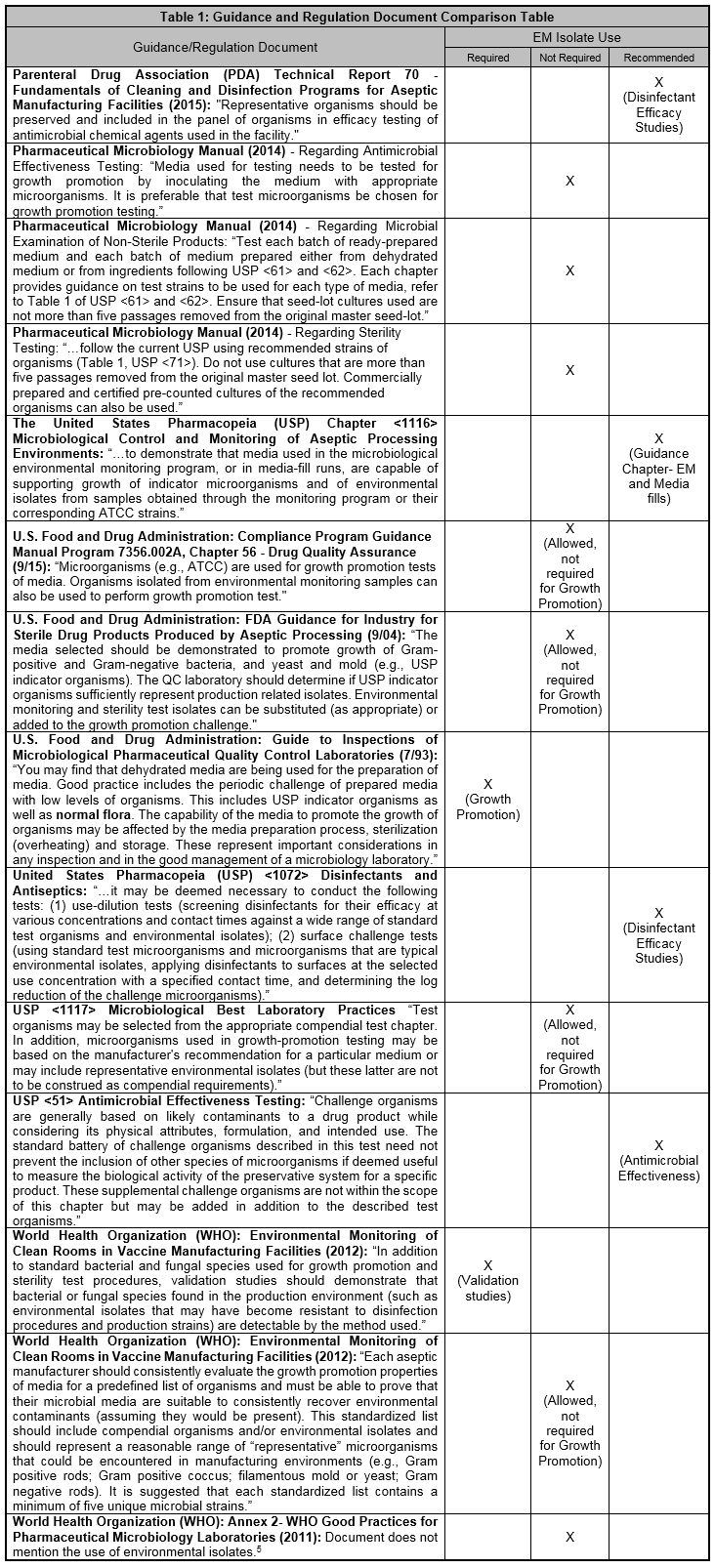
As demonstrated in the table, most literature recommends or allows the use of EIs but does not require the use of EIs in microbial testing. Out of the documents researched, only the FDA Guide to Inspections of Microbiological Pharmaceutical Quality Control Laboratories from 1993 and the WHO document on Environmental Monitoring of Clean Rooms in Vaccine Manufacturing Facilities from 2012 require the use of EIs for growth promotion tests and validation studies, respectively. Interestingly, the FDA’s 2014 Pharmaceutical Microbiology Manual does not require including environmental isolates in testing.
When I spoke to one of the authors of the FDA Guide to Inspections of Microbiological Pharmaceutical Quality Control Laboratories from 1993, this author clarified that the term “normal flora” used in the 26-year-old document was meant to refer to clinical human microbiota and not environmental isolates as we think of them today. The 2014 Pharmaceutical Microbiology Manual is a more contemporary document and is more indicative of the FDA’s current consensus view on the use of EIs in microbiological testing.6
Regulatory Expectations
The regulatory expectation that environmental isolates are incorporated into microbiology testing is gaining momentum. Tim Sandle, Ph.D., head of microbiology at Bio Products Laboratory, points out that “discussions at conferences and symposia suggest that U.S. and European regulators are asking whether environmental isolates are included in release tests and validation and are expecting a justification if this is not the case.”7
This regulatory expectation can be demonstrated in observations issued by the FDA. The following subset of observations regarding environmental isolates have been cited by the FDA.
- FDA warning letter dated Oct. 29, 2010: “Your firm does not perform challenge testing to the sterility media with environmental isolates from the environmental monitoring program.” 8
- FDA warning letter dated May 25, 2011: “The firm was unable to provide scientific rationale for use of the selected organisms used in the Disinfectant Efficacy Study. These organisms were not representative of organisms isolated from the facility nor were they representative of the USP guidelines.”9
- FDA warning letter dated March 1, 2013: “Aseptic processing areas are deficient regarding the system for cleaning and disinfecting the room and equipment to produce aseptic conditions… Additionally, the corporate studies did not evaluate isolates from the [Redacted] site.”9
- FDA warning letter dated Aug. 29, 2018: “Your firm did not have suitability studies that demonstrated that your media procedures and practices were acceptable for microbiological testing of your customers’ OTC drug products and components. For example: Your firm did not perform quality control testing on [Redacted] prepared media to ensure the media support growth and acceptable recovery during testing. You lacked a program that includes quality control testing of all prepared media for its quality attributes, such as pH, and growth promotion prior to use in testing customers’ OTC drug products and components. Our investigators observed that you did not have any microorganisms stored at your facility and did not have the test strains and specified microorganisms for completing microbiological testing. You were not able to provide purchasing records for any reference microorganisms or test strains.”9
I searched the top 100 pharmaceutical companies10 for warning letters related to the use of environmental isolates. I also searched warning letters issued by the Office of Manufacturing Quality between 2015 and 2019 regarding the use of environmental isolates.9 I did not note any additional warning letters that specifically mentioned a failure to use environmental isolates in the growth promotion test or other microbiological assays. This does not mean there are no warning letters written on the topic, just that my search criteria did not uncover any applicable warning letters.
From the warning letters that addressed the use of environmental isolates, one can conclude that there is indeed a regulatory expectation to use them in some microbiology laboratory testing applications, and that expected use appears to include growth promotion of sterility testing media and disinfectant efficacy studies.
The Debate
Like other concepts in microbiology with unclear guidelines, not all microbiologists agree with the practice of using EIs in all microbiological assays. Some microbiologists believe that there “is a strong argument that environmental isolates are the best challenge to culture media as part of release testing and for validation studies like sterility test validation.”7
Other microbiologists believe that once an environmental isolate is cultured for use in laboratory assays, it is technically no longer an environmental isolate. The isolate can adapt easily to its new cultured laboratory environment and will behave differently.
There is also an argument that the EIs were recovered from the environment using the same types of media that will be challenged with those isolates.11 In other words, it has already been proven those microorganisms can grow on the media because those isolates were recovered using that media in the first place. Microbiologists of this mind-set theorize that adding EIs to the growth promotion challenge is a waste of time and money. EIs should only be added to the growth promotion challenge if it makes scientific sense — not because it is a trendy topic in the industry. The use of EIs in growth promotion challenge of sterility testing media is still up for debate, and opinions vary depending on which expert you ask.
Most microbiologists tend to agree that EIs have their place in the laboratory and should be used and handled appropriately. The assays where EIs should be included are the disinfectant efficacy study, microbial assay validations, and antimicrobial effectiveness testing.
The decision to add EIs to the growth promotion challenge test is not a regulatory compliance aspect. “The QC laboratory should determine if USP indicator organisms sufficiently represent production related isolates. Environmental monitoring and sterility test isolates can be substituted (as appropriate) or added to the growth promotion challenge.”12 However, data does not exist that scientifically supports regulatory agencies enforcing the use of EIs in growth promotion tests.11 This decision should be made by the laboratory, and the rationale for deciding which environmental isolates to include in microbiological assays should be established and documented. One practice of choosing environmental isolates is to trend the recovered isolates, determine which microorganisms are the most predominant in the facility, and then use a scientific rationale to decide which microbial isolates are appropriate to include in the assay.
Conclusion
Guidance documents are not clear regarding the appropriate use of environmental isolates in microbiological assays. However, a regulatory expectation for the use of EIs in the microbiology laboratory appears to be gaining momentum.
Not all microbiologists agree regarding the use of EIs in the growth promotion challenge test. However, most microbiologists agree that EIs do have a place in the laboratory. Proper use of EIs in the laboratory includes adding them to disinfectant efficacy studies, microbial assay validations, antimicrobial effectiveness testing, and sterility testing media growth promotion assays. The rationale for deciding which environmental isolates to include in microbiological assays should be established and documented based on a formal risk assessment.
To remain compliant and avoid regulatory observations, follow the established compendial chapters, regulatory guidelines, and execute assays properly according to established SOPs. It is also recommended to stay abreast of growing industry trends and regulatory expectations.
Peer Review:
Special thanks to Tim Sandle, Ph.D., Dennis Guilfoyle, Ph.D., and Adam Hankins for their thoughtful insights and peer review of this article.
References:
- United States Pharmacopeia (USP) <51> Antimicrobial Effectiveness Testing
- United States Pharmacopeia (USP) <61> Microbial Examination of Nonsterile Products: Microbial Enumeration Tests
- United States Pharmacopeia (USP) <62> Microbial Examination of Nonsterile Products: Tests for Specified Microorganisms
- United States Pharmacopeia (USP) <71> Sterility Tests
- Sandle, Tim, Ph.D.- peer review comments via email on June 6, 2019.
- Guilfoyle, Dennis E., Ph.D. (former FDA employee) — personal communications via phone on June 10, 2019.
- Sandle, T. (2018) Microbiological Culture Media: A Complete Guide for Pharmaceutical and Healthcare Manufacturers, “Chapter 9: The Use of Environmental Isolates in Pharmaceutical Microbiology”, DHI/PDA, Bethesda, MD, USA pp. 219-239
- Moldenhauer, J. (2017) “Establishing a Library of In-House Isolates”, IVT Network. Accessed on May 30, 2019 at http://www.ivtnetwork.com/article/establishing-library-house-isolates.
- Food and Drug Administration (FDA) Warning Letters. Accessed on May 30, 2019 at http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/
- Scrip 100- The Worlds Top 100 Pharma Companies by Drug Sales. Access on May 30, 2019 at https://scrip.pharmaintelligence.informa.com/-/media/editorial/scrip-100/2018/league-table/s100-2018_01scrip-100_league-table_online.pdf?la=en&hash=DEEA854FEE9CB726831AB413DA04645C353C9223
- Salaman-Byron (2019) “Facts about Environmental Isolates and Growth Promotion Test”, American Pharmaceutical Review. Accessed on May 30, 2019 at https://www.americanpharmaceuticalreview.com/Featured-Articles/359629-Facts-about-Environmental-Isolates-and-Growth-Promotion-Test/.
- United States Food and Drug Administration: Guidance for Industry (2004) - Sterile Drug Products Produced by Aseptic Processing - Current Good Manufacturing Practices. Accessed on May 30, 2019 at https://www.fda.gov/media/71026/download.
About The Author:
 Crystal M. Booth, M.M., is a regional manager at PSC Biotech and has over 20 years of experience in pharmaceutical microbiology, environmental monitoring, and quality assurance. She obtained her master’s degree in microbiology from North Carolina State University. Booth is a seasoned, award-winning technical writer and author of Method Development and Validation for the Pharmaceutical Microbiologist. During her career, she has worked in microbiology, consulting, quality assurance, CDMOs, R&D, and quality control laboratories. Crystal has developed and validated numerous microbial methods and has worked with many different product types.
Crystal M. Booth, M.M., is a regional manager at PSC Biotech and has over 20 years of experience in pharmaceutical microbiology, environmental monitoring, and quality assurance. She obtained her master’s degree in microbiology from North Carolina State University. Booth is a seasoned, award-winning technical writer and author of Method Development and Validation for the Pharmaceutical Microbiologist. During her career, she has worked in microbiology, consulting, quality assurance, CDMOs, R&D, and quality control laboratories. Crystal has developed and validated numerous microbial methods and has worked with many different product types.
