End-To-End mRNA DS And DP Manufacturing Processes
By BioPhorum

Advanced therapies based on genes, tissues, or cells offer groundbreaking new opportunities for the treatment of incurable diseases. Within these therapies, the use of mRNA offers some unique advantages over other modalities, such as DNA, viral vectors, or recombinant proteins. mRNA manufacture is a cell-free process, which reduces the timeline of production and makes it relatively simple to produce. This promise of an effective, fast, and revolutionary treatment has increased the demand for mRNA production while adding emphasis to streamlining the manufacturing process of new products.
Although product manufacture and process validation of biotherapeutics are performed routinely in the industry, the process for mRNA manufacture is less established and there are several variations between manufacturers.
This article presents the approaches to manufacturing mRNA encapsulated in lipid nano particles (LNPs) across different unit operations for plasmid linearization, drug substance (DS) manufacture, and drug product (DP). Due to the complex nature of each step, we have only included brief details of the four processes involved.
Linearization Of Plasmid DNA
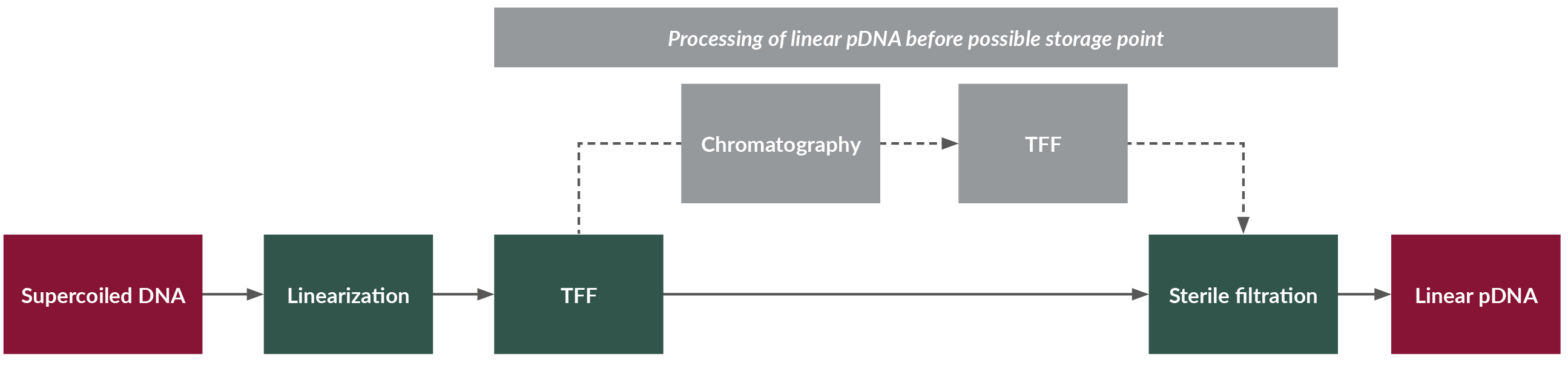
The first step in the mRNA/LNP production process is to produce linear plasmid DNA (see Figure 1).

Figure 1: Plasmid linearization; process flow diagram for linearization of circular plasmid DNA. Linear DNA is a raw material for the n vitro transcription (IVT) reaction. Click on flow diagram to enlarge.
Plasmid DNA Linearization
In this step, circular plasmid DNA (pDNA) starting material is linearized through an enzymatic reaction. Linearization is performed to help prevent readthroughs during transcription of the DNA to RNA in downstream processing steps. The pDNA, a restriction enzyme, and the reaction mixture buffer are incubated in a jacketed mixer or bioreactor. The buffer composition, temperature, pH, mixing speed, and duration of the reaction are chosen and optimized based on the restriction enzyme, DNA sequence, and equipment.
Tangential Flow Filtration (TFF)
TFF is used at two points during a typical mRNA manufacturing process. Generally, TFF is used to concentrate (ultrafiltration) or exchange buffer (diafiltration) and helps remove impurities. Standard stainless-steel or single-use skids and pumps can be used for mRNA manufacturing. Disposable TFF filters/membranes are used in the systems. Chemicals included in the buffer matrix depend on the recipe. Typically, buffer components are chosen to stabilize DNA or mRNA.
Chromatography
Chromatography is a widely used purification method during the linearization of pDNA and mRNA manufacturing processes. Chromatography is performed after the plasmid is linearized and serves to purify/separate linearized pDNA from the enzymes, intact plasmid, or other impurities/residuals used during the linearization step.
Sterile Filtration
Sterile filtration is used to remove biological impurities and contaminants at three points in the typical mRNA manufacturing process: following linearization, following in vitro transcription (IVT), and following LNP encapsulation. Also, sterile filtration may be required as part of onward processing for some products. Manufacturers may choose to use a single filter for this unit operation, especially for a system that is primarily closed.
mRNA Manufacture (DS Generation)
Dilution/Concentration Of pDNA Template: Optional Step
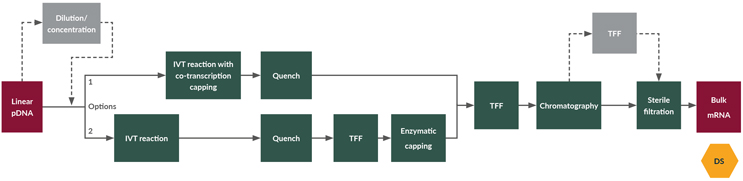
A linear pDNA template is a critical raw material input and its quality should be carefully monitored and controlled. A high-quality linear pDNA template ensures the manufacture of high-quality RNA DS. While a linear pDNA template is charged at a target concentration in an IVT process (see Figure 2), the incoming pDNA template may be too concentrated or dilute and its concentration may need to be adjusted before the template is charged in IVT.

Figure 2: mRNA manufacture (DS generation); manufacture of bulk mRNA through the in vitro transcription (IVT) process. Two options are considered for the capping step: option 1 is co-transcription capping (during the IVT step) and option 2 is enzymatic capping (i.e., a separate step from IVT). Click on flow diagram to enlarge.
IVT Reaction +/- Capping
IVT has been the preferred process in producing mRNA. It is a cell-free system that has been shown to efficiently produce single-stranded RNA molecules of a desired sequence of varying lengths for biopharmaceutical and therapeutic applications. Following the plasmid linearization step, the linearized DNA template is added to the IVT reaction vessel, followed by the addition of IVT buffer, nucleotide triphosphates, enzymes, and unmodified or modified pseudouridine and for the co-transcription capping reaction system and the co-capping reagent.
Quenching
Quenching is performed to terminate enzymatic reactions by deactivating the enzymes. This process step can prevent any residual enzymatic activities that may lead to undesired impurities. Quenching is done at 37°C for 15 minutes to 1 hour. It is performed by introducing DNase-I to the IVT solution after the desired incubation time to digest the DNA template, thus removing the starting material from the reaction and terminating the reaction. DNase digestion also ensures the control of the residual pDNA level in the final mRNA product.
Enzymatic Capping
For 5’-end capping, the mRNA from the IVT reaction is subjected to a dedicated enzymatic capping reaction and the mRNA should be purified before the capping process. Capping enzymes and buffers are added to the mRNA and incubated at 37°C.
Chromatography
This step serves to purify full-length mRNA from impurities/contaminants, including enzymes, DNA fragments, unreacted nucleoside triphosphates, unreacted caps, and incomplete RNAs. An additional chromatography step may be implemented to further purify the material if needed. Affinity resin and/or ion exchange resins are used at this step. Hydrophobic interaction chromatography is less likely to be used for mRNA purification. Different buffer matrices are screened to optimize purification and improve product quality without compromising yield.
mRNA/LNP Manufacture (DP Generation)
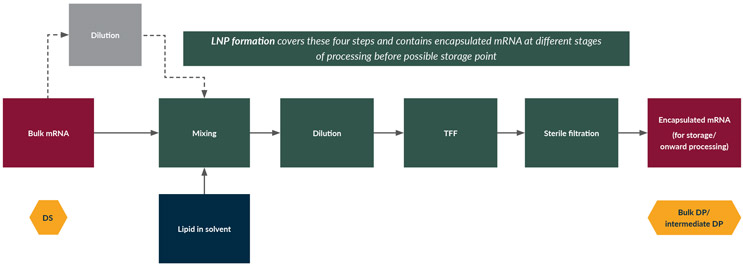
See Figure 3 for the process flow diagram for mRNA manufacture.

Figure 3: mRNA/LNP manufacture (DP generation); process flow diagram for the manufacture of encapsulated mRNA. Click on flow diagram to enlarge.
Dilution
The bulk mRNA is stored frozen in low pH (4 to 6) citrate or acetate buffers at concentrations (typically 0.5 mg/mL to 5 mg/mL) typically higher than what is needed in solutions used for the LNP encapsulation process, and hence it may need additional dilution before the next step. This is achieved by diluting in corresponding low pH (4 to 6) citrate or acetate nuclease-free buffer before the encapsulation process.
LNP Encapsulation Process
Mixing
mRNA/LNPs are formulated by mixing the lipid mixture dissolved in a polar protic solvent with the mRNA stream in an aqueous solvent at a low pH condition to induce spontaneous self-assembly. This is driven by (1) electrostatic interactions between the ionizable lipid and the phosphate backbone of the mRNA, followed by (2) an increase in solution pH to neutralize the ionizable lipid and facilitate hydrophobic interaction as well as changing the solvent polarity to restrict organic solvent in the final formulation. There are many types of mixing techniques, from traditional agitation to in-line techniques such as T-mixing, microfluidics, and x-flow.
Dilution
Following encapsulation, an increase in the pH of the solution is necessary to minimize LNP heterogeneity and polydispersity. Dilution (or neutralization) is performed either by mixing with a high pH buffered solution, phosphate-buffered saline, or by slow dialysis. Slow dilution or dialysis (or dilution and dialysis followed by equilibration hold) is used to provide sufficient time for LNP fusion and particle stabilization and to help the system reach a steady state. After this process step, there is minimal further change in particle size distribution.
Sterile filtration is the final step before the encapsulated mRNA is stored or the production processes is completed and the product is packaged during fill and finish.
Fill And Finish
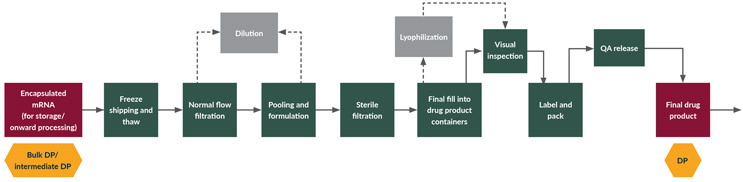
This process step can include a wide range of individual sub-steps (see Figure 4).

Figure 4: Final fill and finish; process flow diagram for the final fill and finish steps to generate final mRNA drug product. Click on flow diagram to enlarge.
Freeze, Shipping, And Thaw
LNP formulations are extremely sensitive to temperature and require cold chain conditions for both storage and shipment. Such a requirement for frozen storage may drive up the manufacturing complexity and cost for LNP DPs and can be a competitive disadvantage.
Normal Flow Filtration
Normal flow filtration is used post-thawing to remove any aggregates that might appear during the thawing step, as these can impact the quality of the DP. At this stage, it is important to choose low-binding and uncharged media filters to optimize product recovery.
Dilution
The product concentration might need adjustment during the freeze-thaw process. An optional dilution step may need to be carried out to reach the right final process condition and concentration of the DP.
Pooling And Formulation
After thawing, the pooling and final formulation steps of the mRNA/LNP DP are ideally performed in closed systems in either small or large tanks. Engineering measures such as temperature control and monitoring of particulate exposure are used to ensure product efficacy and value are maintained.
Sterile Filtration
This step is required for assuring product sterility before final filling into vials. Special considerations might be needed for large-size mRNA/LNP DP that cannot pass through a 0.2 μm pore size of a sterilizing grade filter.
Final Fill Into DP Containers
Filling into the final DP container can generally be done in batch mode or in-line mode. Both scenarios require special considerations for LNPs because of their unique product characteristics. Due to the temperature sensitivity of mRNA LNPs, the in-process manufacturing hold time, i.e., the time when the bulk DP is out of controlled storage, is typically kept low. Minimizing hold times during a batch mode can be challenging, e.g., in the case of large batch volumes.
Lyophilization
Lyophilization, or freeze-drying, is commonly used in the biopharmaceutical industry to preserve product quality, increase stability, extend shelf life, or make the material more convenient for shipping. The process consists of removing water from the formulated product. Lyophilization works by freezing the material, reducing pressure, and adding heat to allow the frozen water in the material to sublimate.
Visual Inspection
Inspecting turbid or opalescent products can be challenging during both manual and automatic inspection processes. The contaminants can reside within the product at the center of the container, thus making it difficult for the inspector or the cameras to detect contaminants. USP <790> specifies a reference time of 10 s/container for the manual inspection process. Due to their turbid appearance, special injectables such as LNPs may require additional time for inspection.
Label And Pack
Labeling of all DPs is required both to provide accurate information regarding the product and to avoid misrepresentation of the ingredients or effects of a drug, whether accidental or intentional. Each separate product, including different strengths or concentrations of the same active pharmaceutical ingredient, must be separately labeled and identified.
QA Release
Releasing a vaccine or therapeutic to the public is a complex process. To make sure vaccines are as safe as intended, assurance of sterility and proper final product quality is essential. Appropriate testing must be conducted to ensure the filled vials, syringes, cartridges, etc., contain the correct quantity of sterile and contaminant-free product and meet the specified product quality criteria.
Conclusion
To meet the rising demand for mRNA products, there is a need for a technology platform and a cost-efficient manufacturing process. Developers and manufacturers must consider the detailed mRNA DS and DP manufacturing processes and any relevant equipment, chemicals, reagents, and parameters required and the scalability of the processes to ensure robust and reproducible manufacturing.
This article summarizes a recent BioPhorum publication on the topic. To read more about the detailed mRNA production process, check out the full paper Overview of end-to-end mRNA drug substance and drug product manufacturing processes and scale-up considerations.