Combining Patient Centricity And Commercial Viability In Pediatric Product Development
By Srinivasan Shanmugam, Ph.D., Adare Pharmaceutical Services

Medication acceptance and adherence are critical concerns in pediatric populations due to these patients’ rapid anatomical and physiological development. Additionally, vastly different pharmacokinetics and pharmacodynamics apply when moving through growth stages from neonates to adolescents. This diversity — combined with a limited market size, a complex formulation process, and stringent regulation — makes the design and development of patient-centric pediatric formulations challenging.
Pediatric Drug Regulation is Evolving Quickly
Before the 1990s, few clinical trials were performed in pediatric patients, and the majority of marketed drugs were not labeled for pediatric use. Therefore, most “pediatric” drugs were administered in an off-label fashion, without adequate understanding of the age-appropriate dosage, formulation safety, or efficacy.
During the 1990s and 2000s, legislation was introduced to mandate specific practices for pediatric medications, and since 2010, there has been substantial review and revision of these guidelines.
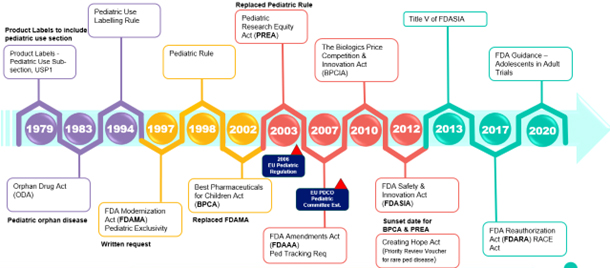
Fig. 1 — Timeline of US and EU regulations impacting pediatric therapies
Particularly impactful rule implementations/changes include the Pediatric Use Labeling Rule in 1994, which required drug manufacturers to survey existing data to support pediatric use info on product labeling. However, this rule was voluntary until the Pediatric Rule was enacted in 1998, mandating adequate pediatric labeling for the approved indication at the time of approval or soon after approval.
The FDA Modernization Act (FDAMA) of 1997 furthered these efforts by compelling the FDA to create a list of drugs for which additional pediatric information would be beneficial. It also introduced a six-month market exclusivity incentive to encourage sponsors to pursue pediatric labeling for more drugs.
In 2002, the Best Pharmaceuticals for Children Act (BPCA) replaced FDAMA, addressing its gaps. The BPCA renewed the exclusivity incentive, established a process for conducting pediatric studies on both on- and off-patent drugs through government contracts, and required that the results of these studies be published or disclosed publicly.
The Pediatric Research Equity Act (PREA) of 2003 replaced the Pediatric Rule. PREA mandates the creation of a pediatric plan that includes a pediatric assessment, timelines, and the development of age-appropriate formulations.
In 2007, the FDA Amendments Act amended and reauthorized both BPCA and PREA, introducing a pediatric review committee and pediatric tracking requirements.
In Europe, EU Pediatric Regulation took effect in early 2007, facilitating the development and availability of medicines for children aged 0 to 17 years. The EU’s Paediatric Committee (PDCO), the EMA’s scientific committee responsible for activities on medicines for children, was around this time as well.
In Europe, the EU Pediatric Regulation came into effect in early 2007, aiming to facilitate the development and availability of medicines for children aged 0 to 17 years. The EU’s Paediatric Committee (PDCO), a scientific committee within the EMA, was established at roughly the same time to oversee pediatric medicine activities.
Combined, the BPCA, PREA, and EU Pediatric Regulation form three important legislative mandates.
Understanding Pediatric Patient Centricity and Needs
The culmination of this regulatory evolution has been an emphasis on the development of age-appropriate formulations for pediatrics. However, for these compliant formulations to succeed commercially, patient centricity in product development is essential. This involves recognizing and addressing the physiological, psychological, and socioeconomic needs of both individual patients and larger pediatric populations within therapy design.
Patient-centric product design is not only comprehensive but also need-based. In this context, age-appropriateness is the primary need. Focusing on this need enhances acceptance and adherence among the patient population, thereby improving therapeutic outcomes.
Pediatric patients are classified as individuals under 16 or 18 years of age (depending on the regulatory agency), and are further divided into five subgroups according to ICH guidelines (Fig. 2).
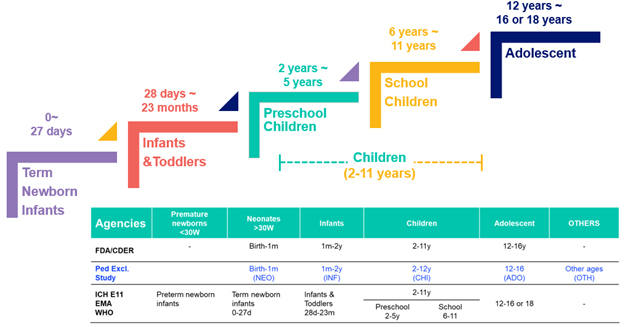
Fig. 2 — Pediatric populations: Product development age (top) vs. pediatric exclusivity study age
The differences between these age groups in product development are profound. Dose requirements can vary by a hundred-fold between neonates and adolescents, making dose flexibility and accuracy critical for age-appropriate formulations. Additionally, a convenient and acceptable formulation is essential. For instance, many excipients can be toxic to pediatric populations, and solid tablets or capsules pose a choking hazard for younger children.
Beyond safety and efficacy, the top priority for pediatric drugs is a palatable formulation. Research and surveys have consistently shown that if a medication tastes bad, children are unlikely to take it. The second priority is swallowability, followed by a lower dose frequency or amount to enhance convenience. Fourth is accessibility, which encompasses packaging and instructions. This ranks fourth because parents, guardians, and caregivers typically manage access to medications for pediatric patients.
Pediatric Product Development Challenges
Pediatric clinical trials are challenging for a number of reasons, with availability topping the list. Given the diverse age groups within pediatric populations, recruitment and enrollment is difficult. Long-term study is impossible because of this patient group’s rapid growth and development — not only in terms of age but also anatomically and psychologically. Additionally, ethical considerations mark every step of a pediatric clinical trial.
Filling these gaps in knowledge is onerous, since ethical issues, patient population heterogeneity, limited blood sampling, and patients’ rapid growth all inhibit studies focusing on pediatric populations.
The next key challenge is bitter-tasting drugs. The perception threshold is very low for bitter tastes (about 0.5 microgram per milliliter) compared to sweet tastes (about 5,000 μg/ml) — a 10,000-fold difference. So, even a small amount of drug with some bitterness will not be accepted by pediatric patients. The conundrum comes from the need to create a highly soluble bitter drug in an easy-to-swallow format, weighed against the possibility that the drug is soluble in saliva, making its perception faster and intense, and unacceptable. This necessitates a taste-masking strategy.
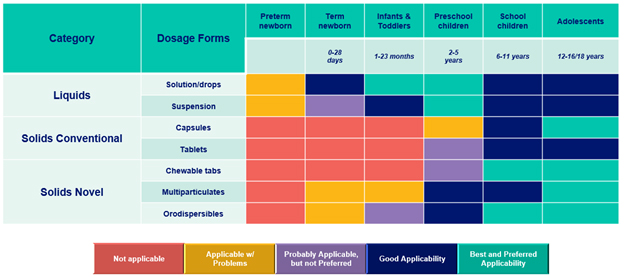
Fig. 3 — Pediatric population dosage form acceptability matrix. Source: “Formulations of choice for the paediatric population,” EMEA/CHMP/PEG/194810/2005.
Next, no single formulation or manufacturing technology offers the flexibility or convenience to meet all pediatric patients’ needs. As noted above, locating and using non-toxic excipients can be challenging, and some drugs may have unpleasant side effects or limited information about their pediatric use.
These concerns intersect with regulatory scrutiny and an approval process that takes longer because of the need for a pediatric study plan (PSP). Finally, even if you achieve a compliant, age-appropriate pediatric formulation, the market share and revenue are always smaller compared to adult products. A balance must be carefully struck between economic viability and regulatory pressure/incentives to work on pediatric products.
Pediatric Product Strategy
The strategy to overcome these challenges starts with improving dose convenience through taste masking and easy-to-swallow formulations. A variety of taste-masking technologies and easy-to-swallow formats are available, including orally disintegrating tablets (ODTs), chewables, and “sprinkles” (e.g., drug-containing pellets or granules sprinkled onto soft foods). Novel formats also play a significant role, such as slippery coats, swellable gel forms like Adare’s Parvulet®, and Harro Höfliger’s Xstraw.
Next is dose flexibility and accuracy. Various technologies, particularly multiparticulates, address this need effectively. Mini tablets, pellets, granules, and beads can be used to create a powder for constitution, with or without taste-masking. For accurate dosing, unit packaging or dosing devices like calibrated scoops, spoons, or oral syringes, as well as multiparticulate or mini tablet dispensers, can be employed based on the patient's requirements. Multiparticulates can also be converted into liquid formulations or direct-to-mouth applications, enhancing dose flexibility, accuracy, and convenience.
As experts in age-appropriate pediatric formulations, Adare offers several multiparticulate-based technologies to meet client needs. Microcaps® is our taste-masking platform based on the coacervation (phase separation) process. Optimum® is a precision particle fabrication technology able to produce microspheres and microcapsules. Diffucaps® is our fluid bed Wurster coating, and Minitabs™ can be produced as 1.2-mm-to-3-mm mini and micro tablets. Finally, Duragran® is our modified granulation process for better particle size control.
Each technology can be created with taste-masking as well as customized drug release. Additionally, all these formats can be converted into reconstitution suspensions, orally disintegrated tablets, chewables, or sprinkles, enabling convenient dosing and optimizing patient acceptability. In short, all these platform technologies offer patient-centric, age-appropriate dosing solutions.
Overcoming A Pediatric Development Challenge
An Adare customer had developed a pancreatic enzyme product (PEP) for the treatment of exocrine pancreatic insufficiency (EPI) due to cystic fibrosis (CF) or other conditions. However, the customer faced several challenges associated with this product, including:
- Infants and young CF patients have difficulty swallowing several capsules of a pancreatic enzyme product a day, requiring convenient.
- A broad dosage range was required (3,000 to 25,000 USP units) to achieve optimal symptom control and precise dosing.
- Format size was limited to 1.5 mm for pediatric patients to accommodate dosing through sprinkle beads and gastric tube feeding.
- Most importantly, the enzyme needs to be protected from stomach acid from degradation.
In response, Adare put the PEP into enteric-coated, (pH sensitive polymer coating) taste-masked minitablets. These were filled into capsules, to be sprinkled later onto soft foods based on the dose requirements, which were in turn based on body weight or age. In doing so, Adare met the client’s need for convenient and accurate dosing while offering the flexibility of dosing.
Patient-Centric Formulation Drives Acceptance and Adherence
The pediatric population is heterogeneous and has specific needs for medication acceptance and adherence. The key factors for achieving this are dose convenience—particularly palatability and swallowability—as well as dose flexibility and accuracy.
Multiparticulate-based technologies provide all three attributes and help overcome various challenges in pediatric product development, such as excipient selection, clinical studies, and regulatory hurdles. Adare’s extensive expertise in pediatric development and its technological solutions offer significant benefits to sponsors aiming to develop and successfully commercialize age-appropriate pediatric products.
Combining patient centricity and commercial viability in pediatric product development is essential. By prioritizing the unique needs of pediatric patients while leveraging advanced technologies, we can create effective, safe, and marketable medications that improve therapeutic outcomes and enhance the quality of life for children worldwide.
To learn more, contact the author or visit https://adarepharmasolutions.com.
About the Author
Dr. Srinivasan Shanmugam has over 20 years of experience in designing and developing conventional, NDDS/alternate, advanced/modified drug delivery systems, and pharmaceutical platform technologies for oral and other routes of administration. His expertise includes stability, solubility/dissolution, permeability, and bioavailability enhancement techniques/technologies for challenging drugs, as well as patient-centric solutions focusing on pediatric and geriatric populations to achieve dose convenience, flexibility, and precision.
In his current position, Dr. Shanmugam is involved in the development and expansion of Adare's pharmaceutical technology portfolio and supports product development, co-development, and tech transfer opportunities. He holds a Ph.D. and a B.S. in Pharmacy. Dr. Shanmugam has published numerous research articles, holds multiple patents, and serves as a reviewer and editorial member for various prestigious journals. His recent work focuses on product development solutions for special patient populations, such as pediatric and geriatric populations.
About Adare Pharma Solutions
Adare Pharma Solutions is a global technology-driven CDMO providing end-to-end integrated services, from product development through commercial manufacturing and packaging, with small molecule expertise focusing on oral dosage forms. Adare’s specialized technology platforms provide taste masking, customized release, solubility enhancement, and patient-centric dosing solutions. With a proven history in drug delivery, Adare's seven facilities in the US and Europe have developed and manufactured more than 65 products sold by customers worldwide.
