Best Practices For Designing Microbiology Experiments
By Tim Sandle, Ph.D.
Qualifying microbiological methods is not easy, compared with analytical ones. This partly rests with the relatively high variation inherent in many methods, especially the culture-based ones.1 While it is unlikely that microbiological methods will meet the more exacting demands of their chemical counterparts, a sound scientific approach can be taken for running experiments and “qualification” of microbiological methods.
This article looks at factors to consider in drawing up assessment criteria for a microbiological test, including the limit of detection (for example, what is the lowest level of microorganisms that can be detected?), specificity (for example, what range of different microorganisms can be detected?), and quantification (for example, the counting accuracy). These types of questions should form the basis of a microbiological method’s validation strategy.2
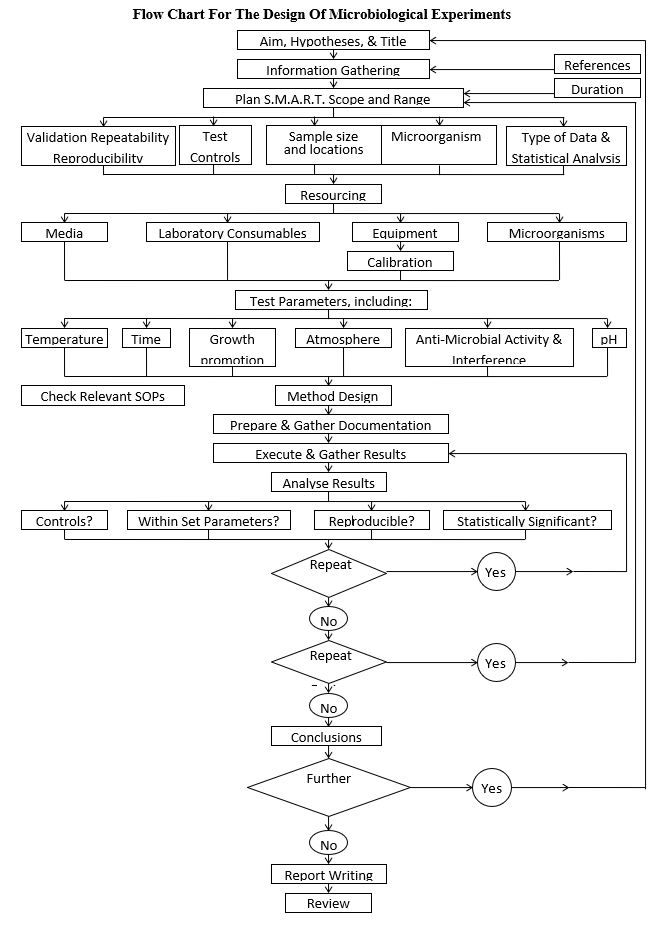
There are several aspects of experiment design that need to be considered and incorporated into the microbiological study design stage. Some of these are displayed in the diagram below, which provides a flow chart for a generalized approach to experimental design.
Samples
The size of the test sample must be considered. Importantly, the samples must be representative and of a sufficient number. A statistical technique may be used to set the number of samples required. Care must be taken here since the statistical technique selected may influence the sample size. The appropriate volume of sample may be a factor, particularly with bioburden testing and ensuring that the sample tested is representative of the final homogenous bulk.
For most validation exercises, the number of batches tested is three (or more). With areas like environmental monitoring, consider where the samples are to be taken, such as the number of locations within a cleanroom.
The following should also be considered:
- testing samples at the end of the shelf life or expiry time (this may include assessment at interim time points)
- degrading samples stored in containers
- holding samples under “worst case” conditions (such as upper or lower temperatures), and
- testing samples at the end of any required process hold times.
The above points are applicable to many bioburden and bacterial endotoxin tests.
Whether any prerequisite treatment of the sample, e.g., neutralization or promotion of reproduction of microorganisms, is needed should be considered.
Test Controls
Experiments should normally have duplicate positive controls, negative controls, and, where recovery needs to be demonstrated, positive product controls (direct product challenges or “spikes”). The level of recovery must be defined at the outset and the level of recovery must be justifiable in terms of experimental aims and the test method employed.
Microorganisms
An experiment normally requires a range of microorganisms. For all experiments, microorganisms from an approved culture collection should be used to ensure uniformity and traceability. In some cases, compendia will indicate the types (or event-specific strains) of the microorganisms required (for example, the sterility test chapter within the main pharmacopeia). In other cases, the microorganisms will need to be selected based on professional judgment. Experiments may be supplemented by environmental isolates, or “wildtypes,” as appropriate (normally, when a culture medium is used to monitor a production process).
In selecting microorganisms, it is often a good idea to draw these across a range of different morphological types. Suitable categories include:
i) Gram-positive rod and/or a Gram-positive spore-bearing rod, e.g., Bacillus sp.
ii) Gram-positive cocci, e.g., Staphylococcus aureus
iii) Gram-negative rod, e.g., Pseudomonas aeruginosa
iv) Fungi (yeast), e.g., Candida albicans
v) Fungi (filamentous), e.g., Aspergillus brasiliensis
In all instances, either the specific culture collection reference must be quoted or the source of the isolate identified. For certain Gram-positive bacteria, the protocol should specify if the organisms should be in the endospore state.
The type of experiment must be considered when allocating microorganisms. For example, a project involving the examination of water would most likely require a Gram-negative rod (such as Pseudomonas sp.) and a coliform (such as Escherichia coli). In contrast, an experiment conducted at 55°C (as with a test for thermophilic microorganisms) would involve a thermophile such as Geobacillus stearothermophilus. Consideration should also be given to the storage of cultures.
Sometimes microorganisms are not recovered as expected. This is a case for carrying out method development work in advance. An example of poor recovery can occur with Gram-negative rods. With these organisms, desiccation can occur during aerosolization. This may occur when using an active air sampler or following loss of moisture content in an exposed settle plate surface. This effect tends to damage Gram-negative bacteria more greatly, because soluble cell contents tend to leak and mechanisms to control the transfer of molecules and ions in and out of Gram-negative cells in particular are considerably impaired. Damage to the mucopeptide lipopolysaccharide center cell structure also causes cell damage and loss of viability. Such damage occurs very shortly after weight loss to a medium or after aerosolization. Such cell damage is typically irreversible.3
Microorganisms should normally be prepared from cultures that are no more than 24 hours old and no more than five passages from the seed lot. However, some microorganisms require longer cultivation than 24 hours. In these instances, the culture age must be documented in advance to ensure that the culture used is as young as it can be. With passages (or subcultures), this is to prevent phenotypic variations from occurring, which might influence the way the microorganism behaves in the presence of the sample.4
It is good practice to confirm the purity of cultures in advance. This can be done either by acceptance of a certificate of analysis from the supplier of the cultures or by confirmatory identification conducted by the recipient laboratory. In some circumstances, such as when conducting a challenge test, a post identification confirmation may also be deemed necessary.
With some experiments, an attempt may be made to induce a stressed state to the microbial population. This will need to be decided at the time the test protocol is written. The reason for attempting this is that the “unstressed” batch culture grown organisms is an artificial creation that rarely exists outside the laboratory.5 Stress factors faced by microorganisms in the environment include:
- Desiccation
- Nutrient deprivation
- Nutrient limitation
- Cold shock
- Heat shock
- Exposure to ultra-violet light
- Other types of radiation leading to sublethal damage
- Responses to disinfectant or detergent residues
- Responses to preservative residues
- Osmolality.
Creating a stressed state is difficult in itself and difficult to verify, given the unknown effects of causing damage to cells or of suppressing cellular growth. In addition, the age of a culture will affect its recovery and will be important for certain identification methods.
Temperature
Consideration needs to be given to the temperature ranges at which the experiment needs to be performed. Here, there is little value in restricting validation to one temperature range for testing that takes place over multiple ranges. An example would be testing culture media at 20 to 25oC and then using it across the range 20 to 40oC and expecting its growth promoting properties to be consistent. Commonly used ranges include:
- 2°C - 8°C
- 20°C - 25°C
- 30°C - 35°C
- 36°C - 38°C
- 55°C - 60°C
In addition to the above, dual incubations across two or more ranges may be required (such as for the enumeration of bacteria and fungi with a single-use culture medium for environmental monitoring).6
Time
As with temperature, the period of time over which the study is to be conducted must be taken into account. Getting this right is important since the selected time becomes the maximum run time for the test. It is important to ask whether the incubation time is set long enough to show the limit of detection. The time of the validation read must never exceed the time used by the testing laboratory for the reading of samples.
Moreover, in specifying incubation times, the minimum time must be clearly stated. For example, you would specify “incubate plate at 20 to 25oC and read at 24 and 48 hours,” rather than “incubate plate at 20 to 25oC and read after 48 hours.” The acceptable tolerance should be stated. Is “read at 48 hours,” for instance, reading within ±1 hour of 48 hours or will a wider tolerance be used, and with what justification? Depending on the test method, it may be appropriate to time samples going into and out of incubation.
Incubation times should be realistic in terms of loss of viability or loss of growth supporting properties of media (e.g., the time set should not be so extensive that plate media desiccation would occur).
Growth Promotion
A key question here is: What type of growth promoting conditions are required for the cultivation of microorganisms? This would include different types of agars, broths, dilution reagents, and so on. These may require a separate (or first phase) validation.
Atmosphere
Whether the microorganism(s) to recover are obligate aerobes, facultative aerobes/anaerobes, microaerophilic, capnophiles, or obligate anaerobes should be considered. A microaerophile, for example, is a microorganism that requires oxygen to survive but requires environments containing lower levels of oxygen than are present in the atmosphere (that is <21% O2; many require 2% to 10% O2). Once established, it will be known whether a specific combination of gases is required, such as, for example, CO2N.
Antimicrobial Activity/Interference
Note should be taken of whether any of the experimental conditions produce an antimicrobial effect or interfere with the test through enhancement or inhibition. Attention should be paid to the best method for neutralizing any antimicrobial properties. Common methods for neutralization include dilution, rinsing, filtration, or the use of general or specific neutralizers.
pH
Note should be taken of pH conditions. pH is important to microbial growth and certain ranges will be unsuitable for some microorganisms.
Growth Phase
The growth phase of a microbial population can impact the accuracy of a method, such as, for example, a turbidity method. Ordinarily, in culture, the following growth dynamics are observed:7
- Cells initially adjust to the new medium (lag phase). Cells maybe growing in mass but not in number. Lag phase is influenced by size of the inoculum; time to recover from physical damage; time required for synthesis of essential coenzymes; and time required for synthesis of new enzymes necessary to metabolize the substrates present in the medium.
- Cells then start dividing regularly by the process of binary fission (exponential phase). Here, cells divide at a constant rate, expressed as generational or doubling time. Ideal generation times vary according to different microbial species. Escherichia coli, for example, will double every 17 to 20 minutes, whereas Mycobacterium tuberculosis doubles every 790 to 930 minutes.
- When their growth becomes limited, the cells stop dividing (stationary phase).
- Eventually, cells show loss of viability (death phase).
With other methods, growth is not a requirement and, instead, cell count is of importance. Such methods may or may not be able to distinguish between viable and non-viable microorganisms. A method like flow cytometry, for instance, will count all cells irrespective of whether they are viable.
Number Of Microorganisms
Some methods will require a minimum number of microorganisms to detect them, such as, for example, a method linked to microbial catabolism, adenosine triphosphate (ATP) growth, bioluminescence, etc. A pre-growth preparatory step may be required to maximize recovery.
Single Cultures
Most methods require a single (pure) culture to obtain a valid result. Mixed cultures can be employed in certain circumstances, such as, for example, container closure integrity. Here, care must be taken that one microorganism does not significantly outgrow the other. In general, mixed cultures should be avoided.
Enzymes
When detecting the presence of specific microorganisms using a selective medium, the specificity and selectivity of the microorganism and media must be demonstrated using selective and non-selective microorganisms.
Summary
This article presents an overview of the validation of microbiological methods, considering some of the limitations and outlining the key criteria that may be applicable for assessment. This review is additionally useful given the more exacting requirement for rapid and alternative microbiological methods.
The extent to which any of the described criteria are suitable will depend upon the sophistication of the method and the limit quantification or detection required. These are outlined here for the microbiologist to consider. Once relevant criteria are selected, they can be built into a validation protocol and the experimental execution will stand up better to regulatory scrutiny.
This article has been adapted from chapter 4 of the book Digital Transformation and Regulatory Considerations for Biopharmaceutical and Healthcare Manufacturers, Volume 2, written by Tim Sandle and co-published by PDA and DHI. Copyright 2021. All rights reserved.
References
- Sandle, T., Leavy, C., Jindal, H. and Rhodes, R. (2014) Application of rapid microbiological methods for the risk assessment of controlled biopharmaceutical environments, Journal of Applied Microbiology, 116 (6): 1495-1505
- Newby, P. (2005) Implementation, validation and registration of rapid microbiological methods. European Pharmaceutical Review. 13(3): 67-73
- Friedman, E.M.; Warner, M.; Shum, S.C.; Adair, F. (2014) In-Process Microbial Testing: Statistical Properties of a Rapid Alternative to Compendial Enumeration Methods. PDA Journal of Pharmaceutical Science and Technology. 69(2): 264-269
- Anderson, J D (1966): Biochemical Studies of Lethal Processes in Aerosols of E.coli, Journal of General Microbiology, 45: 303 – 313
- Snell, J.J.S. (1995) Preservation of Control Strains. In J.J.S. Snell; D.F.B. Brown; and C. Roberts. (Editors). Quality Assurance: Principles and Practice in the Microbiology Laboratory; London: Public Health Laboratory Service, pp 69-76
- Gray, J.C.; Staerk, A.; Berchtold, M.; Hecker, W.; Neuhaus, G.; and Wirth, A. (2010) Growth promoting properties of different solid nutrient media evaluated with stressed and unstressed micro-organism: Prestudy for the evaluation of a rapid sterility test. PDA J. Pharm. Sci. Technol. 64 249-263
- Dupont, C. and Augustin, J. (2009) Influence of stress on single-cell lag time and growth probability for Listeria monocytogenes in half Fraser broth App. Environ Microbiol. 75: 3069-3076
About The Author:
 Tim Sandle, Ph.D., is a pharmaceutical professional with wide experience in microbiology and quality assurance. He is the author of more than 30 books relating to pharmaceuticals, healthcare, and life sciences, as well as over 170 peer-reviewed papers and some 500 technical articles. Sandle has presented at over 200 events and he currently works at Bio Products Laboratory Ltd. (BPL), and he is a visiting professor at the University of Manchester and University College London, as well as a consultant to the pharmaceutical industry. Visit his microbiology website at https://www.pharmamicroresources.com.
Tim Sandle, Ph.D., is a pharmaceutical professional with wide experience in microbiology and quality assurance. He is the author of more than 30 books relating to pharmaceuticals, healthcare, and life sciences, as well as over 170 peer-reviewed papers and some 500 technical articles. Sandle has presented at over 200 events and he currently works at Bio Products Laboratory Ltd. (BPL), and he is a visiting professor at the University of Manchester and University College London, as well as a consultant to the pharmaceutical industry. Visit his microbiology website at https://www.pharmamicroresources.com.

