Assessing Quality Of mRNA Vaccines: Key Considerations
By Diane McCarthy, USP
The COVID-19 pandemic has resulted in the approval or authorization of an unprecedented number of novel vaccines in a very short time. As part of the rapid response to the pandemic, several relatively new vaccine platforms emerged as leading modalities, including mRNA vaccines.1 While research on the mRNA platform has been taking place for many years, the urgency to respond to the COVID-19 pandemic required rapid development of large-scale manufacturing and the adoption of quality control tests to ensure safe and effective vaccines. With multiple manufacturers making similar vaccine modalities and with contract manufacturers playing a key role in expanding production capacity, a variety of analytical tests for mRNA quality checks have been established.
Given the urgency to respond to the pandemic, the limited supply of COVID-19 vaccines, and the novelty of the mRNA platform, many countries receiving vaccines approved in other countries bypassed in-country acceptance testing in favor of accepting testing results from the country of origin. However, as the world moves to a more normal pace of vaccine development and distribution, it is important for global stakeholders to align on what quality looks like for mRNA vaccines. Since the successful widespread application of this technology is relatively new, there are limited regulatory guidelines and industry standards to guide mRNA vaccine quality control during development and manufacturing.
The lack of standards is a challenge for the development of pharmaceutical products, but it is particularly precarious for vaccines because they are intended for otherwise healthy people. A successful vaccination campaign depends on a vaccine manufacturing process and control strategy that minimizes variability and impurities that might cause adverse effects because any severe reactions diminish public support and participation.2 The necessary quality, both at scale and across multiple manufacturers, can only be achieved with standards. Therefore, developers, manufacturers, regulatory agencies, and national control laboratories need publicly available guidelines, methods, and materials to help them to assess the critical quality attributes of vaccines.
To address the need for standards for this new modality, the United States Pharmacopeia (USP) has convened vaccine experts to identify important quality attributes for mRNA vaccines and to propose test methods that can be used to assess mRNA vaccine products. The resulting draft guidelines, Analytical Procedures for mRNA Vaccine Quality, have been published for public comment and feedback. In addition to sharing these guidelines at the World Vaccine Congress in April 2022, USP also hosted a pre-conference workshop in March titled “USP Standards to Support Quality of COVID-19 Vaccines for the Global Community” with industry experts. This article summarizes the product quality attributes and proposed methods that can be used to ensure vaccine quality.
Quality Attributes Of mRNA Vaccines
There are many different types of RNA-based therapeutics, including antisense oligonucleotides, aptamers, small interfering RNA, and microRNAs, but it is messenger RNA (mRNA) that has become a leading modality for vaccines, as well as therapeutics to treat a variety of diseases, including cystic fibrosis and several cancers.2 One of the largest benefits for companies that choose an mRNA vaccine platform is the realization of previously unimaginable manufacturing timelines. In the past, building a commercial-scale production process for a new vaccine could take up to three years. During the COVID-19 pandemic, however, manufacturers were able to choose an antigen, optimize its genetic code, and generate clinical batches of their mRNA vaccines within weeks of the publication of the SARS-CoV-2 genome. The use of a cell-free process with single-use platforms that eliminated the downtime for purification and maintenance contributed to the accelerated timelines. In addition, all these steps can be accomplished in a single GMP manufacturing facility, eliminating added time and risk associated with shipping drug substances between facilities.
Once this process is up and running for one mRNA vaccine, it can be used to produce vaccines against new targets (novel variants or new diseases) with only a minimal number of changes to the processes and formulation since the majority of the vaccine critical quality attributes (CQAs) stay the same regardless of the disease target. Many of the same analytical approaches developed for COVID-19 mRNA vaccines can be applied to future vaccines and therapeutics for other diseases. This overlap accelerates the development of new vaccines and therapeutics because it reduces the resources needed for creating in-house standards for quality control.
These benefits depend on the CQAs for the mRNA platform being firmly established, along with appropriate methods and standards to support robust measurement of the CQAs. Even with all the effort dedicated to mRNA vaccines over the last few years, vaccine CQAs and test methods for this platform are not as well-defined as for other types of vaccines. Unlike most other vaccines and biotherapeutics that are produced in cells, mRNA production is cell-free and uses a series of enzymatic and/or chemical reactions. The cell-free manufacturing platform, along with differences in the biochemical nature of mRNA vs. traditional protein-based vaccines, results in several unique mRNA CQAs. USP’s draft guidelines on mRNA quality attributes and test methods will create a shared understanding of mRNA quality attributes overall, which will, in turn, help accelerate product development and strengthen the quality and consistency of the vaccine product. CQAs for mRNA vaccine drug substances are summarized in Table 1.3 A few considerations and CQAs are discussed in more detail below.
Table 1. Critical Quality Attributes (CQAs) and Analytical Procedures for mRNA Vaccine Quality
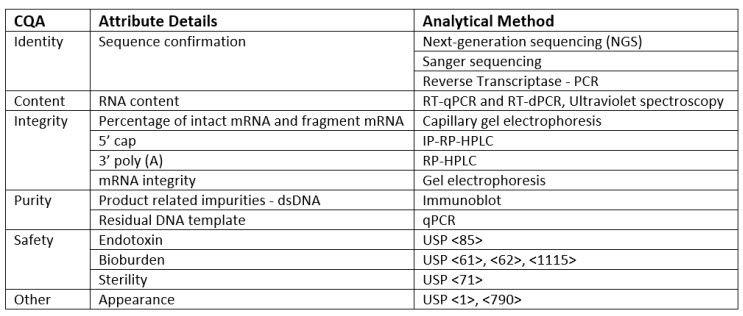
In the context of mRNA vaccine quality control, confirming the identity of the mRNA drug substance is of utmost importance. Confirmation of the identity of the mRNA drug substance is based on analysis of the nucleotide sequence of the mRNA and needs to be able to distinguish between unrelated mRNA as well as any specific changes made to the sequence to address disease variants. A variety of different techniques can be used to assess identity, including next-generation sequencing, Sanger sequencing, and PCR-based methods.
mRNA has several unique structural features. In mRNA vaccine quality control, it is crucial to evaluate the unique structural features of mRNA to ensure the quality and consistency of the drug substance. that must be evaluated to ensure the quality and consistency of the drug substance. Eukaryotic mRNAs carry a 5’ cap that improves mRNA stability and the efficiency of translation to protein. Similarly, mRNAs contain a poly-A tail, which can vary in length and impact stability and protein expression. Both the 5’ cap and poly-A tail length and distribution are important to ensure the quality and consistency of the mRNA and the resulting protein antigen.
Impurities from the cell-free synthesis steps must also be considered. Whereas impurities for cell-based production systems include host cell components, mRNA impurities include raw materials like nucleosides as well as ancillary materials used in the synthesis, such as residual impurities from enzymes, capping reagents, and ethanol used during extraction. Product-related impurities like dsRNA and truncated or incompletely capped mRNAs must also be considered. Ensuring the absence or controlled levels of these impurities is crucial for maintaining the critical quality attributes of vaccines.
The first version of the USP draft guidelines is focused on drug substances, but the mRNA drug substance is not the only component that needs to be considered. The delivery of mRNA vaccines is made possible by encapsulation using lipid nanoparticles (LNPs). LNPs enhance mRNA stability, promote intracellular delivery and release, and give the developer more granular control over immuno-recognition and cellular tropism. Therefore, the encapsulation efficiency of the mRNA is also a vaccine CQA with several parameters that need to be analyzed, including interfacial charge, lipid content/composition, encapsulation efficiency, and the conformation of the mRNA. However, LNPs have no precedent in pharmaceutical manufacturing, so approaches and analytical methods for assessing their CQAs are still evolving. While analysis of LNPs was not included in the first draft of the guidelines, it is under consideration for a future version based on public comments.
Potency is another vaccine CQA for the mRNA drug product and was not covered in the initial draft guidelines. While potency tests will be specific to a given product, potency broadly depends on two functions: uptake of the mRNA by the cells and translation of the mRNA into the encoded antigen. Potency is typically evaluated by measuring protein expression in cell lines or in vitro translation of mRNA in cell-free assays. There remains some debate about the role of potency assays for mRNA vaccines. While mRNA potency assays are not necessarily efficacy-indicating, in vitro assays are generally considered useful for assessing manufacturing consistency and vaccine stability. Given the novelty of the mRNA vaccines, knowledge of how physicochemical characteristics and function in vitro reflect potency in vivo is still evolving.
For example, one challenge discussed during the March 7 webinar and panel discussion was lipid degradants and impurities that can modify the mRNA sequence. 4 Lipid-modified mRNA will fail functional assays that evaluate gene and protein expression, but the modification occurs at such a low incidence rate that it will appear normal when analyzed by most standard analytical techniques, so specific analytical methods for mRNA vaccine quality are needed to detect lipid-modified nucleosides.
Building A Consensus
Public standards serve as the foundation for a robust global quality environment that assists manufacturers by increasing the predictability and reliability of their production processes. However, new vaccine platforms are plagued by a lack of consensus on CQAs, limited information on testing strategies, and a lack of standardized methods. To address these challenges, USP is working to identify mRNA analytical procedures and best practices that support quality assessments for mRNA vaccines throughout development and manufacturing. Achieving this goal requires a great deal of collaboration, which is why USP has recruited dozens of independent volunteer experts from academia, industry, and regulatory agencies to share their diverse knowledge and perspectives on testing strategies, standardized methods, and CQAs for vaccines.
Building the knowledge base and reaching a consensus on vaccine CQAs and analytical methods is a multiple-step process. USP first developed quality assessment toolkits that provide general information on tests that are relevant for vaccines and reference applicable USP General Chapters and other resources. The COVID-19 vaccine quality assessment toolkits for navigating documentary standards that support the development and validation of assays for the evaluation of quality attributes for COVID-19 vaccines are available here. In addition to a toolkit that summarizes tests that are common across all vaccines, there are also platform-specific toolkits for mRNA, viral vectored, inactivated, and subunit vaccines available at that hyperlink.
USP’s platform-based guidelines support the testing of the vaccine’s critical quality attributes with step-by-step procedures to support the quality assessment of mRNA vaccines. Since the launch of the mRNA draft guidelines in February 2022, a wide variety of comments have been received from manufacturers, regulators, vendors, testing labs, and academic researchers worldwide, which USP will evaluate for inclusion in the next iteration of the guidelines.
Resources
- Verdecia, M. et al. COVID-19 vaccine platforms: Delivering on a promise? Human Vaccines & Immunotherapeutics, 1-21, doi:10.1080/21645515.2021.1911204 (2021).
- Azarpanah, H., Farhadloo, M., Vahidov, R. & Pilote, L. Vaccine hesitancy: evidence from an adverse events following immunization database, and the role of cognitive biases. BMC Public Health 21, 1686, doi:10.1186/s12889-021-11745-1 (2021).
- Damase TR, Sukhovershin R, Boada C, Taraballi F, Pettigrew RI, Cooke JP. The Limitless Future of RNA Therapeutics. Front Bioeng Biotechnol. 9. (2021) doi:10.3389/fbioe.2021.628137
- Packer, M., Gyawali, D., Yerabolu, R., Schariter, J. & White, P. A novel mechanism for the loss of mRNA activity in lipid nanoparticle delivery systems. Nat Commun 12, 6777, doi:10.1038/s41467-021-26926-0 (2021).
About The Author:
 Diane McCarthy, Ph.D., is senior director, science and standards in USP’s Global Biologics Department. Over the past few years, Diane has led a team that focuses on development of new standards that can help address common bottlenecks in the biotechnology industry across diverse areas, including vaccines, monoclonal antibodies, and cell and gene therapy. She also oversees development and maintenance of documentary and physical reference standards for biologics. Prior to joining USP, Diane worked for several small CROs that focused on the use of mass spectrometry for characterization of biologics, host cell proteins, and biomarkers.
Diane McCarthy, Ph.D., is senior director, science and standards in USP’s Global Biologics Department. Over the past few years, Diane has led a team that focuses on development of new standards that can help address common bottlenecks in the biotechnology industry across diverse areas, including vaccines, monoclonal antibodies, and cell and gene therapy. She also oversees development and maintenance of documentary and physical reference standards for biologics. Prior to joining USP, Diane worked for several small CROs that focused on the use of mass spectrometry for characterization of biologics, host cell proteins, and biomarkers.
