Analytical Testing Strategies For Clinical And Commercial Operations
By Bikash Chatterjee, CEO, Pharmatech Associates
 According to Medpace, overall outsourced drug development spending is expected to grow to $31 billion in 2019. As the complexity and characterization of new drug therapies increase, so does the challenge for organizations to accommodate technical, capital, and resource requirements.
According to Medpace, overall outsourced drug development spending is expected to grow to $31 billion in 2019. As the complexity and characterization of new drug therapies increase, so does the challenge for organizations to accommodate technical, capital, and resource requirements.
Central to every drug development program is the need to establish a capable analytical testing strategy. The complexity of the drug development marketplace has continued to escalate, moving from small molecules to large molecules and those that include complex molecules — such as cellular and gene therapy products — resulting in enormous technical challenges associated with managing such a broad diversity of analytical testing requirements. Couple this with the expansion of partnering and in-licensing as a core component of pipeline development, and the necessity for a cohesive analytical testing strategy has never been greater.
GATHERING INFORMATION
To better understand how the industry is dealing with these issues, Pharmatech Associates developed a survey to determine what viable options exist for drug manufacturers to use internal development and outsourcing testing strategies to address the technical and business performance requirements for small, large, and complex large molecules and regenerative medicine drug modalities.
We polled seven pharmaceutical and biotechnology companies to understand the factors considered when establishing an analytical testing strategy for clinical material release. Of the seven companies included in the survey, six had mature small molecule programs in addition to large molecule programs. Five of the seven are primarily biotechnology organizations that have small molecule and complex molecule development programs. One is a virtual biotech organization with no internal manufacturing capabilities. While the results of the survey are highly detailed, we will examine several main conclusions from our analysis of the findings.
ANALYTICAL TESTING CONSIDERATIONS: DRUG MODALITIES
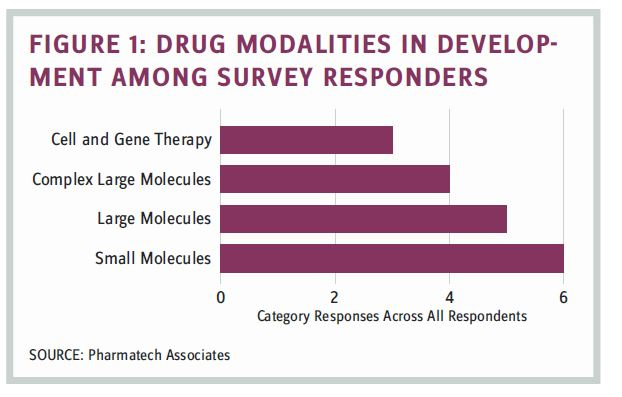
The survey focused on identifying factors that entered into the analytical testing strategy, including organizational responsibility for oversight and development, factors that influenced internal versus external testing, and factors driving an internal versus external testing strategy. We sought to understand if the drug modalities presented any unique challenges that would influence the structure and decision-making regarding testing support. Respondents were asked to provide specific insight on the basis of their current testing strategy for each drug modality. Distribution of drug modalities managed by the survey responders is shown in Figure 1.
FACTORS INFLUENCING ANALYTICAL TESTING
Each respondent was asked how they determined their current analytical testing strategy through the following open questions:
a. Are there different strategies or resource models pertaining to internal versus external division of responsibilities for method development, validation, testing, and release of clinical materials for Phase 1 to Phase 3 clinical materials?
b. Specifically, respondents were asked to choose from a list of factors that influenced the current testing strategy as it relates to alleviating operational pain points:
- capability/core expertise
- capacity
- resources
- geographic coverage
- lead time
- quality systems/compliance
- adaptable for changes from Phase 1 to Phase 3
- cost-effectiveness/fee for service
- flexibility
Results for each of these questions are shown in Figure 2.
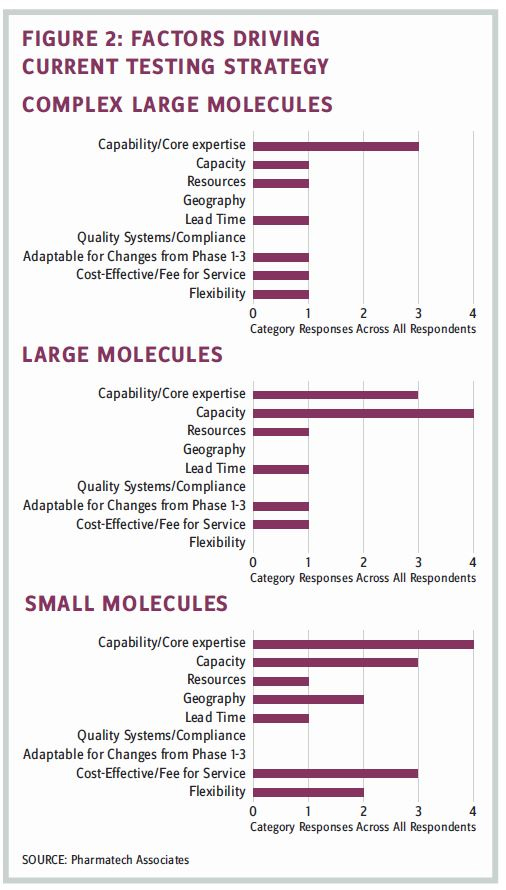
Capability and capacity were significant considerations regarding whether the current strategy employed an internal versus an outsourcing approach. Cost and flexibility also factored into the small molecule testing strategy.
FUNCTIONAL RESPONSIBILITY FOR DEVELOPMENT AND TESTING
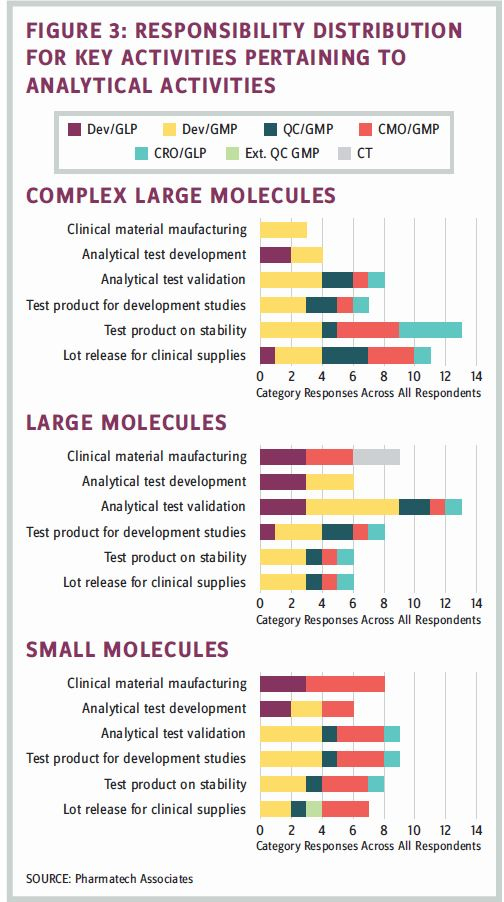
Each respondent was asked to describe how the key activities associated with analytical testing work. The results, based upon drug modality, are shown in Figure 3.
Many of the organizations in the survey were biotech companies, and their indigenous testing expertise was not a core element of their in-house expertise. Large molecule testing was internally supported by the development group, which was largely responsible for core analytical activities.
FACTORS DRIVING AN INTERNAL TESTING STRATEGY
Large molecule manufacturing showed the strongest evidence of relying upon internal testing resources of the three drug modalities. The factors underlying the decision to maintain internal testing varied according to drug modality, as follows:
- Many CMOs lack available capacity and are either eliminating or do not offer analytical testing services, thereby complicating the manufacturing and testing logistics.
- One respondent indicated it was moving away from project-based contract service provider engagements in favor of a fee-based full-time equivalent (FTE) model to address the available capacity shortfall. No other respondents indicated they had moved in this direction yet.
- Insufficient method development was a factor. CMOs can slow down method transfer to GMP/QC labs for later-stage clinical-material-release testing.
- There is a strategic commitment to manage GMP risk for Phase 2 and 3 clinical material by testing internally.
- Analytical resources are bundled with bulk drug substance (BDS) and DP (drug product) process development (BDS is heavily analytical).
- Release testing is linked to the stability-testing program that is often managed internally.
FACTORS DRIVING OUTSOURCING STRATEGY
Both small molecule and large complex molecule manufacturers had greater dependence on outsourcing. Small molecule testing used outsourcing to a much greater extent than large and large complex molecule programs, partially because there are many CMOs and contract-testing labs providing mature, proven testing capability and capacity. Large complex molecule manufacturers sought to utilize a greater percentage of outsourcing support primarily because the complexity of many of these analytical tests required external expertise not always resident within the organization.
When large molecule programs looked to outsourcing testing services, the decision was driven by a strategic desire to consolidate platform programs, which today revolve around monoclonal antibodies (mAbs) in common with external resources, thereby leaving internal resources with the capacity to address new program needs.
QUALITY MANAGEMENT SYSTEM (QMS) FRAMEWORK
Respondents were asked whether they shared a common QMS with their commercial quality group or if there was a phase-appropriate QMS employed for products in development.
Half of the respondents indicated they have a single QMS covering clinical and commercial testing operations. This QMS structure has common policies with SOPs specific to development, clinical, or commercial operations.
The remaining organizations utilized separate QMS systems to address development/clinical and commercial testing operations. Respondents emphasized that organizing a QMS during clinical development required creating a QMS that supports flexibility and risk-based thinking.
SURVEY CONCLUSIONS
The following conclusions were drawn from the survey data, regardless of the drug modality:
- All organizations place the primary responsibility for clinical trial material testing and release with the development organization. In several cases, the quality control organization was responsible for testing; however, this was primarily a resource- and capacity-driven decision, with development still maintaining overall responsibility for testing and release.
- Core competency was a primary factor in determining whether the activities were insourced or outsourced. Fundamentally, if the knowledge was not resident in-house, the work was outsourced.
- For organizations with a developed outsourcing program, cost-effectiveness and timeliness were the key factors in the decision to outsource, although there was no specific evaluation criteria or confirmation of the cost savings targets shared by respondents.
- Large complex molecule development was the only drug modality where testing was consistently outsourced to manage the investment in capital and resources until drug viability was established clinically. This consideration was not cited for small and large molecule development.
- More than 70 percent of the respondents maintained responsibility for method improvement and method changes within the development function: This organizational responsibility was insensitive to the drug modality.
IN CONCLUSION: QUALITY AND LIFE CYCLE MANAGEMENT MATTER
Examining the information from the survey, several models emerged regarding responsibility for life cycle management of the release test method. For most companies the commercial quality function manages and maintains the release-test methods during the product life cycle. The task is accomplished often in collaboration with development and development QC groups for any significant changes or the development of alternate methods.
In some organizations, a mixed model was applied, with development being accountable for the science and technical elements of the method, under a stewardship model, and with quality being accountable for the operational elements, namely, method execution, QC-QC transfer, and obsolescence. This model integrates the rigor derived from quality oversight, which can be advantageous in the case of an accelerated regulatory program.
BIKASH CHATTERJEE is president and CSO at Pharmatech Associates.
