A Portfolio Approach To Achieving Excellence In Pharmaceutical Manufacturing
By Booma Yandava, KPMG 
The pharmaceutical industry has traditionally been guaranteed relatively high ROI and profit margins from blockbuster products, thereby enabling investments in drug R&D rather than in operational efficiencies or cost-containment measures. However, increased global demand for less expensive generics and complex biologics, along with complexities in producing and delivering qu ality therapeutic products, has resulted in a shift in pharmaceutical product portfolio, manufacturing, and supply chain strategies.
ality therapeutic products, has resulted in a shift in pharmaceutical product portfolio, manufacturing, and supply chain strategies.
In addition, pharmaceutical companies wishing to do business in emerging markets are being pushed to revisit their supplier and collaboration configurations, as well as invest in local manufacturing facilities — a strategy of “in country, for country.” As a result of these changes, there is clearly a need to elevate manufacturing and supply chain management to more strategic and tightly integrated roles. Such elevation can be achieved by applying a portfolio approach wherein synergies between the product portfolio, supply chain models, and manufacturing processes are aligned to anticipate demand and quickly adjust output capacity. This paradigm is already being implemented in the technology and retail industries, where product-specific fulfillment and manufacturing are becoming more commonplace.
Portfolio Approach — One Size Does Not Fit All
A supply chain portfolio approach to achieving manufacturing excellence is not a “one-size-fits-all” process of converting materials into therapeutic agents via a monolithic, siloed, replenishment-based supply chain. Rather, it is achieved when manufacturing takes responsibility for productivity and process innovation, and responds to global demands by closely aligning with supply chain strategies, driven by the company’s long-term strategic and financial goals for optimal performance. The best place to start is with the product portfolio.
Understanding the Product Portfolio
Given the highly dynamic nature of the pharmaceuticals industry, business strategies shift, as do their product categories and their associated complexities for manufacturing. Understanding and evaluating a company’s product portfolio for core and noncore fit and aligning sources of value creation with the delivery process are both key to understanding how supply chain and manufacturing choices can be evaluated.
For instance, manufacturing and order fulfillment demands on biologics are not the same as those of generics or branded therapeutics. While current models tend to limit the analysis of products to demand forecasts, clinical trials, manufacturing costs, price, and other parameters, products must be evaluated in the context of a product portfolio for manufacturing and fulfillment needs. Further, a product portfolio must be assessed for product design, supply chain response requirements, manufacturing flexibility, and financial goals.
Examples of such criteria include:
- product design and packaging considerations, such as product shelf life, dimensions, quality, and supply base that will feed into the bill of material (BOM)
- order-to-delivery needs for each product line, product mix, region, and customer segment to evaluate speed versus predictability
- customer/partner integration
- cost considerations and asset management goals to align with overall strategic and financial goals
- determination of commercialization and manufacturing characteristics early on during the new product introduction stage for complex products
- a robust integrated business planning model to support product portfolio management, product commercialization, supply chain portfolio, and manufacturing.
The Supply Chain Portfolio
Biologics are characterized by low-volume, disease-defined small batch sizes, and shorter lead-time. The make-to-order (MTO) fulfillment model, along with demand-driven capacity planning, can be leveraged for manufacturing such therapeutic agents. Batch processes with single-use equipment can be adapted to biologics for niche markets where scalability is not a factor. Batch processing also makes it easy to switch production of one product to another, either for clinical trials or sustained manufacturing. Biologics are more amenable to a responsedriven fulfillment model that favors on-time delivery, personalized customer service, and flexible supplier contracts.
Generic products, with their mix of high volume and low variety, can be fulfilled by leveraging make-to-stock (MTS) or make-toforecast (MTF), since batch sizes are large and products can be customized for a given market. Manufacturing follows smooth production with low-cost sourcing strategies. Demand forecast, product quality, asset utilization, and Lean manufacturing are key considerations to being more efficiencydriven than responsiveness-driven.
On one end of the spectrum is the responsiveness- driven pull model; on the other end is the efficiency-driven push model. A product portfolio may not conform to one or the other supply chain model neatly, due to the nature of the products in a portfolio. For example, biologics will have subportfolios of products based on disease states, delivery mechanism, shelf life, and so forth. Although inventory reduction is ideal, the demand-based pull model is highly complex, and does not always lead to cost reduction in every situation. Degrees of agility, responsiveness, and efficiency trade-offs can be achieved further by applying supply chain strategies such as product postponement, vendor-managed inventory, and other supply chain network considerations where finished goods inventory (FGI) is not owned by the companies until they are shipped out to end customers. A small amount of safety stock is maintained at the regional distribution centers (RDCs) based on the replenishment lead time and variability of demand.
Postponement strategies are specifically useful when local design and manufacturing are desired. Trade-offs between cost and responsiveness determine the best possible supply chain model.
The keys to achieving excellence in pharmaceutical manufacturing and the supply chain are understanding the demand patterns by SKUs and then defining the order fulfillment process for the finished goods. Further, upstream processes, such as raw material (RM) supply chain, procurement, and warehousing, can be decoupled and refined across the spectrum of push to pull to achieve an optimal supply chain model. This can be achieved by conducting a detailed mapping of the fulfillment process from formulation to quality control to packaging and distribution, with a focus on lead time at every node, minimum safety-stock requirements, impact of major events on inventory levels such as approval of drugs, total landed cost, and manufacturing constraints for each product portfolio. Further, the supply chain model can be optimized for resource, transportation, supplier contracts, cross-border delivery, and other criteria.
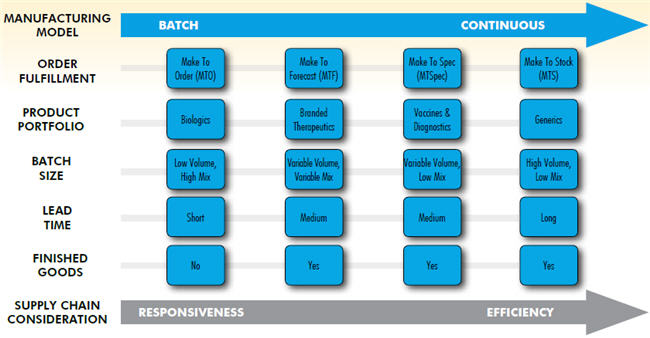
Aligning Manufacturing with the Supply Chain Portfolio
Today’s pharmaceutical manufacturing functions largely as a disconnected business with little alignment with fulfillment models. The right supply chain model for a product or a portfolio of products depends on how well the supply meets the demand.
With increased yield and efficiencies, lower capital and operating expenditure, less waste, and greater product recovery and economies of scale, continuous manufacturing is well suited for generics-based MTS or MTspec therapeutic agents. Continuous manufacturing offers an advantage in that capacity can be adjusted to market demand. Local manufacturing plants can be configured to reduce transportation and stocking to meet local demands. Various manufacturing sites dispersed globally can be brought to the same level of quality standards. Product demand for generics can come from various sources, including online. Therefore, it is imperative that manufacturing be cost-efficient such that order fulfillment is always ready to meet any demand with a cost-effective push model.
Biologics with an MTO fulfillment model are well-suited for batch processes where sales forecasts are unstable, the customer population is small, and product and manufacturing complexities are high. Batch processing enables careful considerations of product modifications, such as accurate posttranslational modifications, safety, quality, and downstream processing. For metabolite-based therapeutic agents, the batch process may be preferable due to strain degradation and stability issues that are often associated with continuous manufacturing technology. Recombinant DNA-based biologics are better produced using batch processes where optimal culture conditions are achieved for improved yields.
Further, a fed-based culture system — a hybrid system between continuous and batch processing — can be adopted where quality, cost, order-to-delivery time, cycle time, and other factors can be easily controlled to align with a selected supply chain model.
Evaluating manufacturing processes and aligning with the product portfolio and supply chain model will reduce complexity and enable optimal capacity utilization. A responsive manufacturing process that avoids the risk of inventory pileups and obsolescence can be achieved by adopting a flexible manufacturing model in which it is easy to switch between a batch process and a continuous process according to the fulfillment model identified based on the product portfolio.
With the recent trend of moving to a continuous manufacturing process, the looming question is: Does adopting one or the other process for all product types and supply chain models yield desirable results?
Defining pharmaceutical manufacturing that extends well beyond production cost and labor arbitrage and that generates value from supply chain fulfillment and product portfolio encompasses the following key elements, at minimum:
- a segmented manufacturing approach such as a ”job-shop factory” for low-volume, highly-differentiated MTO products, and a dedicated production line for high-volume, lowmix, MTS-based products
- a sophisticated modeling tool to simulate various plant configurations to enable testing various plant configurations for varying fulfillment models and product portfolios
- a postponement capability to enable packaging, labeling, and just-in-time delivery of products in a location-specific manner
- standardized business processes to optimize trade-offs among performance, yield, and price
- Engaging manufacturing business units or CMOs from the very product portfolio definition stage.
Guidelines for Excellence
Plant efficiency and ideal full capacity utilization cannot be achieved without tight integration of supply chain processes and a product portfolio assessment, of which new product introduction (NPI) is a key part. Regardless of the supply chain model and manufacturing processes, a visionary manufacturing organization will have shorter cycle time, pull-based API consumption, capacity planning that is closely aligned with NPI planning, tighter finished inventory for goods, order tracing, shipment tracing, integrated quality, and transparency. These are just a few examples of levers to elevate manufacturing execution to a more strategic role. In addition, the product portfolio should be continually renewed with a focus on shorter time-to-market, maximized forecast accuracy, and end-to-end efficiency to ensure responsiveness and efficiency of producing and fulfilling orders.
Cycle time and customer experience are key metrics to track for improving responsiveness, and customer experience is a key consideration for generics or products that are in a steady state. In addition, assessing of inventory levels on an ongoing basis is imperative for cost reduction and efficiency management.
Regardless of the products, place, and manufacturing model, the following are some of the best practice recommendations that can save money and improve margins:
- With increasing costs and lower return on investments, pharmaceutical manufacturing under a one-size-fits-all umbrella is no longer sustainable.
- A portfolio approach that starts with a product portfolio assessment to match manufacturing strategies with the supply chain and fulfillment model will not only decrease inefficiencies and cost but also improve timeto- market in a sustainable way.
- With demand-based pull strategies and a supply-based push model, pharmaceutical manufacturing can become more responsive with clear visibility into the quality of products. External collaboration with suppliers and customers and internal collaboration with product development teams, supply chain operations, sales and marketing will help build tighter links along the value chain to strengthen manufacturing.
Above all, integrated manufacturing planning must carefully consider risks, such as increasing demand uncertainty, exploding product proliferation and complexities, and global uncertainties.
Conclusion
The proposed approach is not without challenges. For example, it is not clear how a continuous manufacturing process characterized by economies of scale can be adopted for a pull strategy. Similarly, Lean, Six-Sigma concepts that are well adopted for a continuous manufacturing process with high-volume, low-mix and repetitive operations lines pose challenges when adopted to biologics-based therapeutics agents.
But with cross-pollination from other industries’ improvements in technology and changing market conditions, pharmaceutical manufacturing can address these challenges and deliver on its parent company’s business and strategic objectives of being responsive and efficient to meet customer needs.
