A Phased Approach To Adopt In-Line Monitoring & Real-Time Release
By BioPhorum

The biotechnology plant of the future will use in-line monitoring (ILM) and real-time release (RTR) as two of the key components to enable the industry’s operational vision. This vision includes process technology, operational systems, facility design, and construction innovations that will increase capacity and enable efficient, sustainable processes, all from a patient-first perspective. It involves advanced process controls (APC), predictive modeling, and ILM/real-time release testing (RTRT) to provide continuous quality assurance, a high level of control over variability, and automated release of product.
This article is a guide to adopting that vision — having precise control and a deep understanding of the manufacturing process in real time such that the amount of release testing required is minimized.
How Do We Currently Release?
Biopharmaceutical manufacturers release through reactive mechanisms in quality systems. This means that any deviations, mistakes, or problems that are found must be investigated and closed after they have occurred. This can take up to several weeks after production and can cause significant delays in the release of product. Other potential issues include:
- A product may have to be rejected if an issue cannot be resolved, resulting in product loss and significant costs.
- Reviewing paper documents introduces the potential for human error and record loss.
- Adding more parties, e.g., contract manufacturing organizations, into the process may result in additional inefficiencies.
Therefore, implementation of ILM/RTR with consistent guidelines is needed to release product more efficiently to the market.
The current QC sampling and testing process is cumbersome and labor intensive, with many potential opportunities for errors in sampling, labeling, transporting, storing, and testing. Using ILM/RTR technologies to eliminate or minimize these opportunities for errors will make the overall manufacturing and “testing” processes easier and more predictable, which translates to a more reliable supply chain and a potential reduction of inventory.
Benefits Of Fully Adopting ILM/RTR
Reduced Time To Market/Commercialization
- Reduced time to market for new, similar products, platforms, and processes due to improvements in process understanding and control
- Deviations detected through real-time feedback by sensors and system (more proactive QA rather than reactive investigations) to improve process understanding and enable better real-time process control, leading to quicker product release
Potential Cost Savings
- Reduced manufacturing costs: decreased sampling materials, potential for longer active ingredient shelf life based on less post-production testing and more real-time testing, reduced time from raw materials to patient, work in progress (WIP) and finished goods inventory
- Decreased capital investments in testing facilities: building, infrastructure, and equipment
- Decreased cost compared to traditional wet-chemistry methods requiring sample collection, solvents, supplies, data reporting, and disposal
- Increased productivity due to fewer deviations and rejections, and more optimized processes
- Decreased inventory (less need for storage space, lower cost of stock, less likely for expired or redundant stock)
- Increased sustainability by reducing or eliminating waste of samples and supplies
Improved Process And Supply Chain Robustness
- Increased production capacity due to shorter waiting times for in-process testing results
- Advanced product and process understanding/control through generation of more data with in-line sensors, while maintaining product safety
- Reduced risk and optimized capacity through QbD with more data available to design reliable, more predictable processes and adjust processes in real time
- Reduced human error associated with sample handling, testing, and manual processes
Digital Maturity And Industry Evolution
- Better-enabled next generation of bioprocessing, for instance, perfusion or continuous manufacturing
- Captured additional benefits in other areas associated with advanced digital maturity, e.g., data availability for additional improvements across products, platforms, and processes
Hurdles To Implementation
The hurdles to implementing ILM/RTR include:
- The technology for large-molecule characterization has not yet been developed for in-line monitoring of all the product quality attributes that are needed for large molecules.
- The technology is not always sensitive or robust enough to replace every assay.
- While regulatory agencies support innovation, they are not harmonized globally. Even though the primary markets and regulatory agencies advocate for more ILM and RTRT, they still have limited experience with both filings and use in manufacturing, leading to extensive questioning.
- Some organizations have struggled to justify investments in ILM/RTR, yet the benefits are significant, and tools are available to aid the justification.
- Some organizations are experiencing change fatigue. Although biopharmaceutical manufacturing is already partly automated, as further advanced technology is adopted there is a risk that manufacturing teams are unfamiliar with new ways of working. Inserting new technology into existing commercial manufacturing sites is difficult and adds complexity.
- Perhaps the biggest challenge is the maturity of ILM instrument vendors and their equipment, which perform well in development with relatively limited use, but once implemented in manufacturing with continuous use, many robustness issues are uncovered.
The complete solution for ILM/RTR will include various components, e.g., instruments and data collection systems, which will all be managed throughout the life cycle of that component as well as through the product’s life cycle.
A Scale Of Adoption Of ILM/RTR
Three strategies that can be used to implement new technologies on the path to ILM/RTR:
- Development – incorporating ILM/RTRT as a new drug product is developed.
- Improvement – adding ILM/RTRT as improvements are made to an existing drug product and process.
- Executive sponsorship – adding a specific new method, technology, or process for a commercial, business, or other reason and adding ILM/RTR simultaneously.
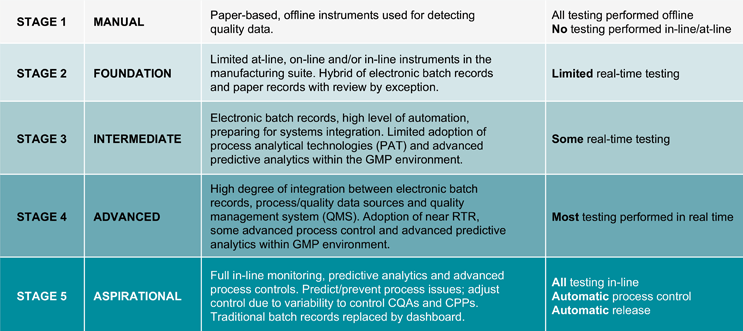
The journey to the biotechnology plant of the future will happen in phases. The first will involve harmonizing processes to reduce variability in the areas of most value. Automated process control and predictable release within shorter timeframes will later become the standard. Finally, automated process control and automatic release will be commonplace, resulting in continuous real-time quality assurance. An incremental scale has been developed to provide SMART milestones for implementation teams and those managing organizational change (see Table 1). The scale can be used in business cases to apply for investment, proposals for proofs-of-concept exercises, and evaluations to report on outcomes. Stage 5 is the highest level of adoption and is aspirational as the technology necessary is not available/sufficiently robust to be implemented.
Table 1: The five stages of adoption of ILM/RTR
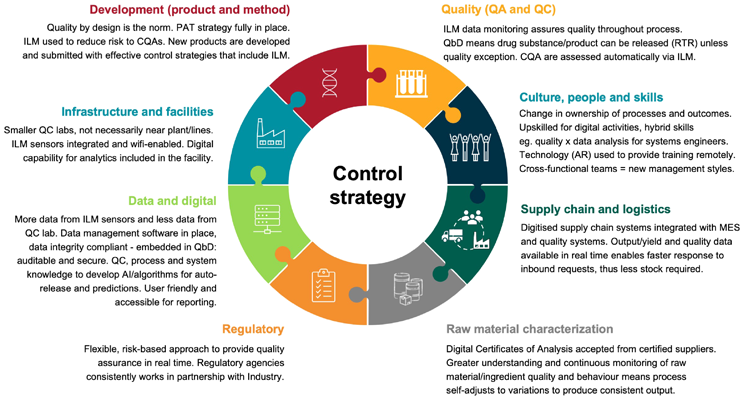
Stage 5 of full ILM/RTR will involve a proactive approach with embedded quality by design, with reduced deviations that are resolved throughout the processing steps. There will be real-time data throughout the supply chain and highly skilled workers who can conduct detailed analyses of that data. To achieve full ILM/RTR adoption, sponsors and stakeholders must be engaged and reach a greater understanding of the long-term vision. The ideal system would be paperless, method-guided automation with electronic templates and workflow software. See Figure 1 for other changes that are needed.
Figure 1: ILM/RTR operational vision – what needs to change to enable smart and adaptive process control? Click on image to enlarge.
Implementation: What Needs To Be In Place?
Organizational Change Management
The process of moving to a higher level of digital maturity begins with leadership determining where they currently are and where they would like to be. A gap analysis identifies what is needed to get to the desired end state, and criteria for determining which product lines are ripe for implementation are agreed upon. At that point, one or more implementation strategies can be adopted and supporting infrastructure put in place. As the process nears implementation, regulatory agencies must be engaged and involved to ensure a smooth path for approval.
Implementing a broad vision such as ILM/RTR requires a dual-pronged strategy — an executive sponsor pushing from the top down and middle management/floor-level employees pushing from the bottom up. All stakeholders must understand where the organization is currently on the path to ILM/RTR and the end goal.
Adopting ILM/RTR will require investments in upskilling people and building their capabilities to interpret data, troubleshoot, maintain instruments and software, etc. These new skills can be built internally using in-house expertise and education or from outside by bringing in new staff with the required expertise.
Relationships With Raw Material Vendors
Although biopharmaceutical manufacturers verify the quality of incoming materials, few use real-time measurements as part of a receiving or dispensing process.
An approach that manufacturers are adopting on incoming materials is vendor/supplier certification. This is when inspection, testing, and release processes at the supplier are accepted by biopharmaceutical companies instead of using in-house testing. This certification process provides an incentive for adopting real-time identification testing at the point of receipt at the receiving warehouse, eliminating the requirement for raw material sampling, sample handling chain of custody, and offline testing.
Data
There will be a steep increase in the volume of data points recorded using ILM. True ILM/RTR will therefore require significant investments in data storage and management. Data collection, contextualization, and retention are important to the success of any implementation and movement up the scale of adoption of ILM/RTR. Importantly, data security and integrity must be preserved at all times, with data transfer and storage being secure and reliable.
The Role Of Industry Standards And Regulatory Guidance
Regulators provide guidance and support for innovation, defining terms such as in-line/online/at-line monitoring and their expectations for these technologies. These documents provide guidance on designing a process control system to allow real-time or near real-time monitoring of critical attributes and identifying and measuring critical material and process attributes. Ensuring control of critical material attributes and having defined process controls (including ILM) supports RTR because the control strategy assures that all critical quality attributes are met. However, to implement advanced control strategies, involving regulators early will be crucial, as well as partnering with them to learn, share, and grow the industry synergistically.
Industry standards create uniformity and acceptance and are already being developed. Regulatory agencies are engaging in dialogue with the industry and soliciting feedback. They see the changes coming and are trying to figure out how they will assess them.
Regulatory bodies are also moving away from requiring exhaustive inclusion of all parameters in submissions and toward predetermination and definition of critical parameters, with selective inclusion in submitted and updated documentation.
Currently, the common regulatory technical document framework requires sections for the justification of release specifications. To use RTR, these would contain a justification for why end product testing is reduced or not required, because the combination of ILM, RTRT, and a tightly controlled process eliminates the need for many of the release tests, if not all of them.
Conclusion
Biopharmaceutical manufacturers have yet to adopt ILM/RTR throughout their operations, but there is a growing appetite for using data to control processes and moving toward release by exception. With growing understanding and experience, companies will gain more confidence, so acceptance and use will build together over time until both manufacturers and regulatory bodies become more comfortable with control strategies using ILM/RTR.
Each organization’s version of “full adoption” of ILM/RTR will differ according to a cost/benefit analysis and long-term investment strategies. Adopting this way of working toward stage 4/5 may remain targeted to certain plants or products within an organization’s range. Ultimate success will be seeing routine global product approvals based on ILM, RTRT, APC, and innovative manufacturing. However, on a smaller scale, some ways to achieve success include reducing product release times (via elimination/reduction of testing and/or digitizing quality systems) and adopting platform technologies that can be implemented and validated quickly and efficiently.
Realizing the vision of true RTR would be transformative for the biopharmaceutical industry, enabling the delivery of products to the market significantly faster than is currently possible, as well as opening up the potential for more life-enhancing products, including personalized medicines.
This article is a summary of a recent BioPhorum publication on the topic. To read more, check out the full report, including case studies, in Operational vision: Adoption of in-line monitoring and real-time release.