A Closer Look At Buffer Recycling For More Sustainable Bioprocessing
By Madelène Isaksson, Ph.D., Lund University

Biopharmaceuticals, particularly mAbs, represent a rapidly expanding segment within the pharmaceutical industry. The production of these biopharmaceuticals involves an upstream process where living organisms are cultivated, followed by a downstream process (DSP). The DSP typically comprises three chromatography steps, virus inactivation, and several filtration steps, all designed to purify the target antibody.1
A notable characteristic of biopharmaceutical manufacturing is its substantial water usage. In comparison to small molecule drugs, the production of biopharmaceuticals requires approximately 10 to 100 times more water per kilogram of product.2 The DSP employs numerous buffers and solutions in large volumes, which are predominantly water-based. The water consumption for the standard mAb manufacturing process ranges from over 3,000 kilograms to nearly 7,000 kilograms per kilogram of product, with the capture chromatography step and the two polishing chromatography steps accounting for over 50% of the total water usage.2
Focus On Productivity Has Ancillary Environmental Benefits
Producing these buffers necessitates high-purity water, buffer salts, specialized equipment for buffer preparation and storage, and skilled operators to prepare and perform quality control on the buffers. Additionally, every liter of buffer used must be disposed of properly.
The high demand for buffers and associated buffer management account for a large part of the process environmental footprint.3 In light of the global commitment to sustainable development and the projected growth of the biopharmaceutical market, the extensive consumption of buffer volumes is anticipated to pose significant challenges.4 Consequently, buffer intensification is becoming an essential aspect of biopharmaceutical production.
Traditional optimization in downstream processing has focused on product purity, yield, operability, and productivity, inadvertently benefiting the environmental footprint.5 For example, shifting from batch to continuous biomanufacturing has enhanced productivity and reduced buffer consumption, while single-use systems have decreased cleaning needs and consequently water usage.6,7
Introducing Buffer Recycling To Further Shrink Consumption
Furthermore, water consumption in biopharmaceutical manufacturing can be reduced through the introduction of buffer recycling. While solvent recycling through re-distillation is well established in solvent-based downstream processing, buffer recycling in water-based DSPs remains relatively unexplored. There are practical examples of buffer recycling occurring, such as in a periodic counter-current chromatography (PCC) process. However, in PCC, buffer recycling is primarily performed to maximize yield rather than to reduce buffer consumption.
We see promising potential in introducing buffer recycling during the chromatography steps of the DSP to address high buffer consumption. Our concept for buffer recycling during chromatography involves two main steps: recovering spent buffer and reintroducing the recovered buffer into the process. The basic idea is to recover buffer fractions that closely match the original buffer quality and then condition the recovered buffer to meet the required properties for reuse.
Figure 1 illustrates the unit operations of a typical mAb DSP. Buffers can be recovered and reused within the same unit operation (option 1) or recovered from one unit operation and reused in another unit operation further upstream in the process (option 2). Since the buffer recycling protocol consists of two steps, its implementation requires a cyclic or continuous process. Therefore, the advancement of continuous integrated biomanufacturing offers numerous opportunities to implement buffer recycling strategies.
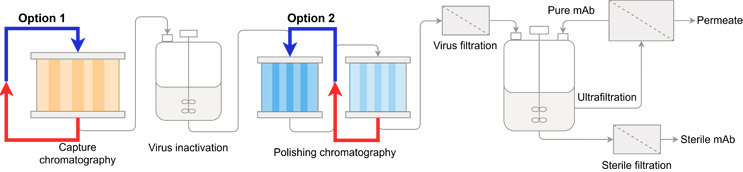
Figure 1: The unit operations of the established downstream process for mAbs, with two options for introducing buffer recycling. Option 1: Buffers can be recovered and reused within the same unit operation. Option 2: Buffers can be recovered from one unit operation and reused in another unit operation further upstream in the process.
Several buffers are used in a chromatographic process, including those for washes, elution, regeneration, cleaning in place (CIP), and equilibration. The choice of buffer to recycle must minimize the reintroduction of impurities into the process, thereby ensuring the final product quality is not compromised. In light of this, wash buffers may be unsuitable to recycle, whereas elution buffers could potentially be recycled, depending on the product and impurity elution profiles.
Recycling equilibration buffers is an interesting option since they are used after the CIP phase, when the product has been recovered, and impurities have already been washed out from the column and system. CIP buffers are another promising candidate for recycling, as they can potentially be reused within the same unit operation or in another unit operation further upstream, given that the impurities present in the used CIP buffers are already part of that process stage.
We have closely examined option 1 of buffer recycling. Specifically, we introduced the recycling of equilibration buffer during the equilibration phase in the capture chromatography step of an antibody within a three-column PCC operation. During the five column volumes (CVs) equilibration phase, the final CVs were recovered at the outlet valve and collected in an intermediate hold-flask. The pH of the collected buffer was measured and found to be slightly above the setpoint, so it was adjusted accordingly, while the conductivity was already at the setpoint. After adjusting the pH, the buffer was reintroduced at the beginning of the next equilibration phase using a versatile valve. Figure 2 illustrates the process setup and highlights the flow paths used during the buffer recovery and buffer reuse steps.
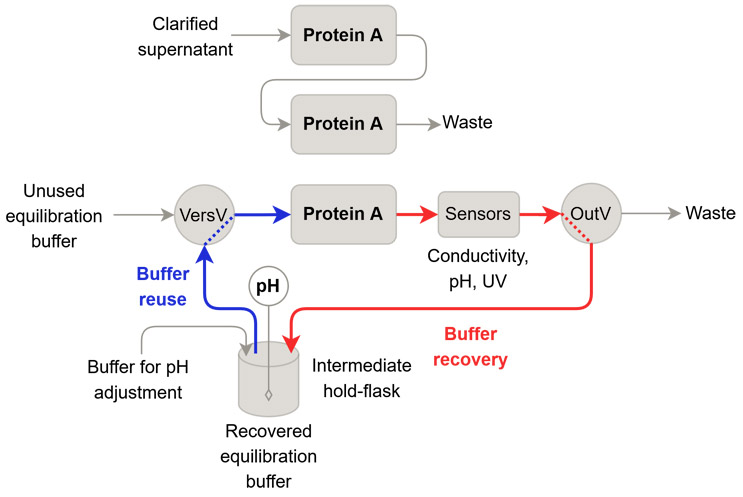
Figure 2: Overview of the introduction of buffer recycling in a three-column PCC process. The flow paths that were used for buffer recovery and buffer reuse are highlighted. In the intermediate hold-flask, a pH sensor was submerged to measure pH. The pH was adjusted by adding buffer for pH adjustment.
By implementing buffer recycling in the equilibration phase, we reduced the consumption of equilibration buffer by nearly 50% in this phase, which corresponds to approximately 10% of the total buffer consumption for the complete capture chromatography protocol. Importantly, we observed no adverse effects on process yield or final product purity. A full description of this work is available in a previous publication of ours.7
Regarding buffer recycling option 2 presented in Figure 1, we propose recycling CIP buffer from the second polishing step to the first polishing step. If the CIP of these two columns is performed simultaneously, depending on the process scheduling, buffer recycling without an intermediate hold-flask could be implemented. The recycling of CIP buffer upstream is still under investigation, but we consider savings of 30% to 50% in CIP buffer consumption as feasible.
Controlling Bioburden And Process Impurities
The quality of biopharmaceutical products is of utmost importance to ensure the drugs are safe and efficacious. All buffers and materials used in the production of biopharmaceuticals undergo rigorous quality control checks. For example, buffers used in the downstream process are tested for pH, conductivity, and sterility before being introduced into the process.
The risk of buffer recycling leading to the accumulation of impurities, such as host cell DNA or proteins, has been debated. However, buffer recycling is an inherent part of the PCC process, through the interconnected wash phase — a process concept already accepted by authorities.
In the presented option 1, we have investigated recycling during the equilibration phase in a three-column PCC operation. Before the equilibration phase, the CIP phase should have removed any adsorbed impurities in the column and system. There is, however, still a risk that some impurities may be present in the recovered buffer fraction. But even if impurities are reintroduced with the recovered buffer, the next step in the PCC protocol is the interconnected wash, which would introduce any possible impurities anyway.
For the recycling of CIP buffers, as proposed in option 2, we assume that impurities do not re-adsorb during CIP conditions. In addition, since we recycle the buffer upstream, the process should be able to handle potential impurities since they are already present in that part of the process.
Another important consideration is controlling the bioburden level in the process. Introducing buffer recycling will require additional equipment and modifications to existing process equipment. However, if the additional systems introduced are of the same standard and are closed systems, the bioburden level of the process should be ensured.
Recycling Reduced Consumption By Nearly Half During Equilibration
Through relatively simple means, we introduced buffer recycling during the equilibration phase of capture chromatography for mAbs. This implementation reduced buffer consumption in that phase by nearly 50%, which is a substantial reduction. Although current process optimization focuses on other areas of downstream operations, buffer intensification, including buffer recycling, may become more significant in the future as the pressure to reduce the environmental footprint of manufacturing processes increases. The transition from batch-based to continuous end-to-end processing is already underway, and buffer recycling can be seamlessly integrated into this shift.
It is time to reconsider the role of buffers in each step of the process. By understanding the purpose of each buffer and conducting a thorough risk assessment for your specific process, buffer recycling could become a viable option for your operations.
References:
- Shukla, A. A., Hubbard, B., Tressel, T., Guhan, S., & Low, D. (2007). Downstream processing of monoclonal antibodies—Application of platform approaches. Journal of Chromatography B, 848(1), 28–39. https://doi.org/10.1016/j.jchromb.2006.09.026
- Ho, S. V., McLaughlin, J. M., Cue, B. W., & Dunn, P. J. (2010). Environmental considerations in biologics manufacturing. Green Chemistry, 12(5), 755. https://doi.org/10.1039/b927443j
- Gibson, K., Oliveira, J. C., & Ring, D. (2023). Evaluation of the Impact of Buffer Management Strategies on Biopharmaceutical Manufacturing Process Mass Intensity. Processes, 11(8), 2242. https://doi.org/10.3390/pr11082242
- Jungbauer, A., & Walch, N. (2015). Buffer recycling in downstream processing of biologics. Current Opinion in Chemical Engineering, 10, 1–7. https://doi.org/10.1016/j.coche.2015.06.001
- Sarkis, M., Fyfe, A. T., Kontoravdi, C., & Papathanasiou, M. M. (2024). Towards a Net Zero, socially sustainable and eco-efficient biopharma industry: How far are we? Current Opinion in Chemical Engineering, 44, 101027. https://doi.org/10.1016/j.coche.2024.101027
- Konstantinov, K. B., & Cooney, C. L. (2015). White Paper on Continuous Bioprocessing May 20–21 2014 Continuous Manufacturing Symposium. Journal of Pharmaceutical Sciences, 104(3), 813–820. https://doi.org/10.1002/jps.24268
- Frank, G. T. (2018). Transformation of biomanufacturing by single-use systems and technology. Current Opinion in Chemical Engineering, 22, 62–70. https://doi.org/10.1016/j.coche.2018.09.006
- Isaksson, M., Andersson, N., & Nilsson, B. (2025). Improving the sustainability of biopharmaceutical downstream processing through buffer recycling. Journal of Chromatography A, 1740, 465545. https://doi.org/10.1016/j.chroma.2024.465545
 About The Author:
About The Author:
Madelène Isaksson recently defended her Ph.D. in chemical engineering at Lund University, Sweden. Her thesis focused on continuous downstream processing of biopharmaceuticals, coupled with buffer intensification, with the goal of making biopharmaceutical manufacturing more efficient, sustainable, and cost-effective. Contact her on LinkedIn.
