Will Transcatheter Valves Continue Cannibalizing Surgical Aortic Valve Replacements?
By Kamran Zamanian, Ph.D., CEO and founding partner, iData Research
Aortic valve disease is one of the most common heart diseases worldwide, with surgical aortic valve replacement (SAVR) as the standard method of treatment. Surgical valve replacement procedures are performed using tissue or mechanical heart valves, and the choice of which valve type to use depends on patient age, disease nature, and other comorbidities. In 2019, Europe saw roughly 85,000 surgical aortic valve procedures, with an 80/20 split between tissue and mechanical valves, respectively.1
as the standard method of treatment. Surgical valve replacement procedures are performed using tissue or mechanical heart valves, and the choice of which valve type to use depends on patient age, disease nature, and other comorbidities. In 2019, Europe saw roughly 85,000 surgical aortic valve procedures, with an 80/20 split between tissue and mechanical valves, respectively.1
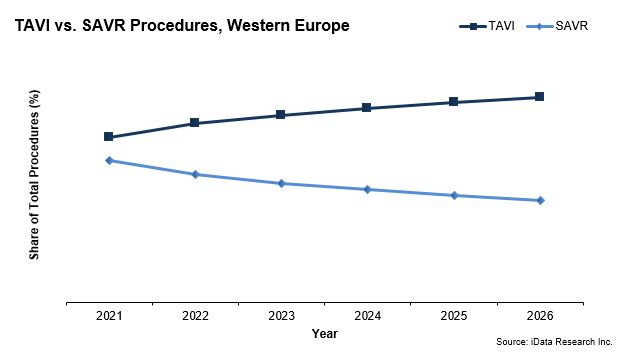
While the surgical aortic valve market size broke €170 million in 2019, it is dwarfed by the transcatheter aortic valve implantation (TAVI) device market, which garnered nearly seven times that amount. TAVI procedures are expected to account for an even larger share of total aortic heart valve replacement procedures in Europe in 2021.
While the overall growth of the surgical heart valve replacement market is being limited by the interest in TAVI procedures, the recent approval for TAVI devices to be used on low-surgical risk patients will impact the debate on choosing a mechanical versus bioprosthetic heart valve.
Increasing the Bioprosthetic Valve Patient Pool
Traditionally, mechanical valves have been preferred for younger patients, as they are designed to be durable over time and often outlast the patient. The recommended age for implanting bioprosthetic valves has dramatically decreased in the past 15 years. In 2007, the European Society of Cardiology recommended the use of bioprosthetic valve for patients older than 65 years of age, and then lowered the age recommendation to 60 years or older in 2012.2 The guidelines of the American Heart Association and the American College of Cardiology suggested that the bioprosthetic valve be preferred for patients over the age of 60 beginning in 2010. An update in 2017 recommended that the age limit be lowered to 50 years of age.3
Drawbacks of Mechanical Heart Valves
While studies show the long-term survival rate between mechanical and tissue cardiac valves are similar,4 one major drawback of mechanical valves is that they contribute to an increased risk of blood clot formation. As a result, mechanical valve recipients often take anticoagulant (blood thinning) drugs, such as warfarin, for the rest of their lives, which makes the patient more prone to bleeding.
For patients taking warfarin, this means living with the associated issues as well, which includes frequent blood draw, drug interaction, activity and diet regulation, and the cost of medication and monitoring.5 However, work is being done to remedy this issue; for example, CryoLife’s On-X mechanical heart valve is CE mark approved with less warfarin than competing mechanical valves. Patients with the reduced blood thinner dose had at least 60% fewer bleeding events without increasing the risk for thromboembolism.
While surgical tissue heart valves do not require an anticoagulant, they are accompanied by a much higher risk of reoperation. A patient with a tissue heart valve may require reoperation if one of the leaflets fails to close, narrowing the valve and forcing blood to leak back into the heart. From a study that looked at patients who underwent aortic valve replacement between 1982 and 2003,6 a 60-year-old man receiving a bioprosthesis had a lifetime reoperation risk of 25% and was unlikely to outlive the bioprosthetic heart valve.
The Impact Of TAVI Device Approval On Low-Risk Patients
The approval for use of TAVI devices on low-surgical risk patients now offers a solution for patients of advanced age who are likely to be poor candidates for open-heart surgical aortic valve implantation. This option is also beneficial for high-risk patients as it allows them to recover in less time — typically one week with TAVI versus three months with a surgical procedure.7
The risk of reoperation still exists, but by using the less-invasive “valve-in-valve” procedure, a new transcatheter valve can be placed into the orifice of a failed surgical valve, pushing the old valve leaflets aside. Thus far, multiple aortic valve reoperations for structural valve deterioration carry an acceptable risk of early postoperative mortality.8
Edwards Lifesciences Pulls Ahead In The Shifting Competitive Landscape
Edwards Lifesciences, Medtronic, Boston Scientific, and Abbott are the four primary companies pushing innovation within the European TAVI market. In 2019, Edwards Lifesciences held the leading position on the TAVI market and managed to gain enough market share to surpass Medtronic. The company saw its European TAVI sales grow as it was the first company to gain CE mark approval for its SAPIEN 3 TAVI device to be used on low-risk patients. While Medtronic fell behind Edwards Lifesciences, it still held over one third of the TAVI device market and is poised to bounce back. Following complications with the LOTUS Edge delivery system, Boston Scientific pulled the struggling product from the market in November 2020 and has now shifted its focus toward its ACURATE neo2 TAVR system.
What’s Ahead For The TAVI Market?
The TAVI market is set to continue developing as new competitors, such as Sahajanand Medical Technology (SMT), JenaValve, and MicroPort, push their devices through the regulatory approval pathway. While COVID-19 has driven a sharp decline in procedure volume in 2020, the market is expected to recover in 2021 and will benefit from strong, persistent growth for the foreseeable future as adoption among the lower-risk patient group picks up.
About the Author
 Kamran Zamanian, Ph.D., is CEO and founding partner of iData Research. He has spent over 20 years working in the market research industry with a dedication to the study of medical devices used in the health of patients all over the globe.
Kamran Zamanian, Ph.D., is CEO and founding partner of iData Research. He has spent over 20 years working in the market research industry with a dedication to the study of medical devices used in the health of patients all over the globe.
About iData Research
For more than 15 years, iData Research has been a strong advocate for data-driven decision-making within the global medical device, dental, and pharmaceutical industries. By providing custom research and consulting solutions, iData empowers their clients to trust the source of data and make important strategic decisions with confidence.
References
- European Market Report Suite for Cardiac Surgery Devices (2020), iData Research Inc.
- Holmes, D.R.; Mack, M.J.; Kaul, S.; Agnihotri, A.; Alexander, K.P.; Bailey, S.R.; Calhoon, J.H.; Carabello, B.A.; Desai, M.Y.; Edwards, F.H.; Francis, G.S.; Gardner, T.J.; Kappetein, A.P.; Linderbaum, J.A.; Mukherjee, C.; Mukherjee, D.; Otto, C.M.; Ruiz, C.E.; Sacco, R.L.; Smith, D.; Thomas, J.D.; “2012 ACCF/AATS/SCAI/STS Expert Consensus Document on Transcatheter Aortic Valve Replacement,” J. Am. Coll. Cardiol. 59 (13), 1200 (March 27, 2012). Retrieved July 10, 2020, from https://pubmed.ncbi.nlm.nih.gov/22300974.
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin III, J.P.; Fleischer, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T.; Rigolin, V.H.; Sundt III, T.M.; Thompson, A.; “2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines,” Circulation 135 (March 15, 2017). Retrieved July 10, 2020, from https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000000503.
- Khan, S.S.; Trento, A.; DeRobertis, M.; Kass, R.M.; Sandhu, M.; Czer, L.S.C.; Blanche, C.; Raissi, S.; Fontana, G.P.; Cheng, W.; Chaux, A.; Matloff, J.M.; “Twenty-year comparison of tissue and mechanical valve replacement,” J. Thoracic Cardiovasc. Surg. 122 (2), 257 (April 23, 2003). Retrieved July 10, 2020, from https://www.sciencedirect.com/science/article/pii/S0022522301620427.
- Kaneko, T.; Cohn, L.H.; Aranki, S.F; “Tissue Valve Is the Preferred Option for Patients Aged 60 and Older” Circulation 128, 1365 (September 17, 2013). Retrieved July 10, 2020, from https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.113.002584.
- Van Geldorp, M.W.A.; Jamieson, W.R.E.; Kappetein, A.P.; Ye, J.; Fradet, G.J; Eijkemans, M.J.C.; Grunkmeier, G.L.; Bogers, A.J.J.C.; Takkenberg, J.J.M.; “Patient outcome after aortic valve replacement with a mechanical or biological prosthesis: Weighing lifetime anticoagulant-related event risk against reoperation risk,” J. Thoracic Cardiovasc. Surg. 137 (4), 881 (February 25, 2009). Retrieved July 10, 2020, from https://www.sciencedirect.com/science/article/pii/S0022522308015729.
- University of Michigan Frankel Cardiovascular Center, “Valve-in-Valve TAVR.” Retrieved July 10, 2020, from https://www.umcvc.org/conditions-treatments/valve-in-valve-tavr.
- Joshi, Y.; Achouh, P.; Menasché, P.; Fabiani, J.; Berrebi, A.; Carpentier, A.; Jouan, J; “Multiple reoperations on the aortic valve: Outcomes and implications for future potential valve-in-valve strategy,” Eur. J. Cardio-Thoracic Surg. 53 (6), 1251 (December 26, 2017). Retrieved July 10, 2020, from https://academic.oup.com/ejcts/article/53/6/1251/4775057.
