What's Next After FDA's DSCSA Extension?
By Carl Accettura, Global Pharma Advisory

It’s been an intense four months of Drug Supply Chain Security Act (DSCSA) preparations and debate over the feasibility of Nov. 27 industry readiness, so it feels like a year since my June guest column on waivers, exceptions, and exemptions (WEE) urgency.
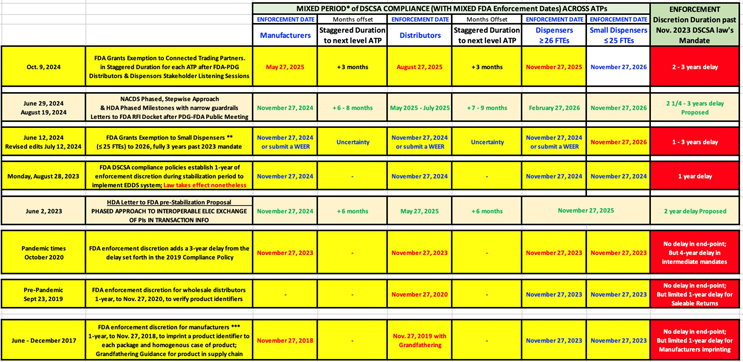
Fortunately, FDA relented this one last time and took a very pragmatic approach by announcing that it will grant staggered exemptions to connected authorized trading partners (ATPs) past the 2024 stabilization period, into 2025, for DSCSA Sec. 582(g)(1) Interoperable EDDS.
Collaboration, communication, systems, and processes remain critically important as industry moves through:
- this next year of implementation, SOP development, and stabilization,
- this mixed period of initial compliance, WEE urgency, and the expected uptick in individual WEEs,
- FDA’s small dispenser exemption for companies with fewer than 25 full-time employees,
- FDA’s Oct. 9 broader, extended exemptions for connected trading partners, staggered through 2025.
After the June FDA-PDG public meeting and stabilization period midway checkpoint, the National Association of Chain Drug Stores (NACDS) and the Healthcare Distribution Alliance (HDA) sent letters in July and August to FDA’s docket RFI recommending a phased, stepwise approach to compliance, but still expecting manufacturer-level trading partners to comply by Nov. 27. Yet, at the same time, NACDS reported hearing that manufacturer-level trading partners were providing accurate, consistent, and complete EPCIS data for only about 25% to 50% of prescription drug products that pharmacies receive and only for some products. NACDS wanted to keep pressure on manufacturers since "the ability for pharmacies to purchase medications from upstream trading partners is completely reliant on the ability of those trading partners to comply with DSCSA requirements."
Consistent with this, Jillanne Schulte Wall, a senior policy director at the American Society of Health-System Pharmacists (ASHP), told Pharmacy Practice News that many ASHP members reported manufacturers are providing only 20% to 30% of the data they need because of cascading enforcement periods, which are preventing an absolute compliance push. “I don’t think manufacturers are trying to be noncompliant; rather, the practical realities of DSCSA have been more complex than people initially expected,” she told the publication.
On Sept. 23, the Louisiana Board of Drug and Device Distributors’ survey of 273 facilities graphically revealed significant gaps in processes, policies, and procedures for identifying ATPs and managing WEEs, using a “Policies & Procedures Coverage Heatmap” to illustrate the gaps. The survey revealed that less than 78% of facilities had policies and procedures that satisfy data exchange, verification, and suspect and illegitimate product compliance areas. Beyond that, only 45% report that they meet compliance for handling exceptions. Considering the “moving target” of WEE preparedness, only 39% reported they had policies and procedures for communicating with trading partners who have a WEE or are otherwise purchasing products exempt from DSCSA requirements.
On Oct. 7, FDA Commissioner Califf received a congressional letter from Reps. Troy Balderson (R-OH) and Lori Trahan (D-MA), co-signed by 19 other House lawmakers, asserting that, “absent government intervention, there will likely be disruptions, drug shortages and patients unable to access critical medications.”
On Oct. 9, after listening closely to industry stakeholders, such as HDA and NACDS, the FDA effectively responded to the concerns of Congress and industry by granting broad exemptions for connected trading partners, reversing prior policy to only grant individual WEEs. This eases implementation complexity and gives these select ATPs more time to build upon the last four years of progress and continue efforts to complete DSCSA requirements by 2025, without disrupting patient access to medicines. FDA believes this broader exemption flexibility is warranted to support DSCSA implementation and lead to a stronger, safer drug supply chain by supporting the select “connected” manufacturers and ATPs (i.e., hard-working, committed ones).
Staggered duration of exemption for “connected trading partners” by categories of eligible ATPs:
- Manufacturers and re-packagers: May 27, 2025
- Wholesale distributors: Aug. 27, 2025
- Dispensers with 26 or more full-time employees: Nov. 27, 2025 (small dispensers: Nov. 27, 2026)
DSCSA Enforcement Discretion Evolution, From 2017 To Now; No Justification For Delaying Efforts
While FDA has applied DSCSA enforcement discretion several times, each relied on a different rationale.
So, do not automatically expect more delays. Elizabeth Gallenagh, the general counsel and senior vice president of supply chain integrity at the Healthcare Distribution Alliance, said “I doubt that anyone is going to have the appetite to move those dates back any further.”
As they did for the stabilization period, FDA once again emphasized that, “The exemptions described … are not intended to provide, and should not be viewed as providing, a justification for delaying efforts … FDA strongly urges trading partners to continue their efforts to implement necessary measures to satisfy these enhanced drug distribution security requirements.”
Keep in mind that it was a once-in-a-lifetime global pandemic that created havoc ahead of enhanced drug distribution security (EDDS) mandates, leading to the unusual 2020 to 2023 deadline delay. Be cognizant of the magnitude of the task of exchanging and managing data for tens of billions of individual drug units and allocate the necessary resources to do the job. Learn from the DSCSA business leaders at PDG, GS1 WG, and HDA who’ve been developing and deploying systems and processes, gaining an appreciation for just how technically complex those systems and process solutions can be. See my tabular analysis below to reflect on how we got here:
FDA Is Not Just Kicking The Can; The Rationale Seems More Nuanced, Being Limited to Already Connected ATPs
Importantly, I say nuanced because the exemption is contingent on an important differentiation of pharma manufacturers that will separate the wheat from the chaff. The exemption only applies to select supply chain “good guys” in my reading, since the “exemption applies to any product transacted by eligible trading partners, which are trading partners who have successfully completed or made documented efforts to complete data connections with their immediate trading partners, but still face challenges exchanging data.”
FDA’s approach is rather consistent with industry’s phased approach proposals, but it importantly holds the goal line at Nov. 27, 2025. It uses a modified staggered duration of exemptions, which differs from the HDA/NACDS proposals of June 2023 and July 2024.
So, let me provide a wake-up call and updated advice for the still demanding DSCSA timelines, following up on my June guest column. Keep your pedal to the metal, since this new May 27, 2025, manufacturers’ finish line is just 7½ months away, which is quite short when it comes to DSCSA EDDS collaboration, testing, feedback, and problem solving across multiple partners.
FDA’s exemption plan recognizes the unprecedented technical granularity of DSCSA interoperability challenges but has only nudged the deadline forward into 2025, and only for select connected trading partners. Manufacturers still need to plan wisely for May 27, 2025, and push extra hard to complete their DSCSA compliance strategy and implementation before this modest breathing room runs out.
Those select ATP good guys who have been working in the trenches over the last 11 years know who has and who has not "successfully completed or made documented efforts to complete data connections … but still face challenges exchanging data." Heck, the FDA even said explicitly that it doesn't need to be notified, so leaders should not obsess over this language. Industrywide during this exemption period, I expect the FDA exemptions can be handled through an "honor system" quite naturally between the select ATPs that are already connected.
After May 27, 2025, if a manufacturer is still not stabilized, then it would presumably need to submit an individual WEE request (WEER), so there is significant incentive to be DSCSA EDDS compliant by then and avoid a WEER. Having progressed with making connections, manufacturers must keep focused on improving the quality of the data being exchanged to cross the goal line and stabilize.
Manufacturers should already have in-house, cross-functional DSCSA initiatives between their internal departments — supply chain, regulatory, and quality. EDDS compliance by your organization still requires your team to conduct external collaboration and coordination and interoperable data exchange testing with your wholesalers (e.g., Cencora, Cardinal, McKesson, etc.) and with your CMOs and their solution providers as well.
In PDG’s July survey results, wholesalers had experienced more varied progress across manufacturers but acknowledged that progress in data exchange between manufacturers and wholesalers has been significant. So, connected wholesalers also get a modest nine months of additional exemption, until Aug. 27, 2025.
Also, in PDG’s July survey results, progress between wholesalers and dispensers has been less momentous, in large part because many of those systems and processes are dependent on wholesalers receiving reliable data from a critical mass of manufacturers. Thus, the dispensers’ exemptions extend out to Nov. 27, 2025 (>26 FTEs) and Nov. 27, 2026 (≤ 25 FTEs).
Recalcitrant Manufacturers Should Expect to Lose Business If They Don't Accelerate Compliance Efforts
As noted above, wholesalers have experienced varied progress across all manufacturers.
In light of this new timeline, effective Oct. 9, this should be a final wake-up call for any recalcitrant manufacturers that have not made sufficient efforts to comply with DSCSA EDDS requirements, even by just connecting. If you have dragged your feet, it’s time to fish or cut bait, since FDA will still be ending its one year of enforcement discretion for manufacturers that are not eligible and connected.
The business risk is that your company’s product will become quarantined and unavailable for sale. FDA’s announcement should light a fire under any non-eligible manufacturers that are not entitled to the extended deadline and will be forced to apply for an individual WEE, which could be uncertain, and therefore could begin to lose business due to their noncompliance. I think they should begin to lose business after Nov. 27.
As Ilisa Bernstein, former FDA DSCSA lead and now president of Bernstein Rx Solutions, aptly noted, "If your trading partner is not ready and is not willing to work with you, then you should seriously consider finding a different supplier."
Enough said. It is now or never for all manufacturers, and it may already be too late for some.
Systems And Processes – SOPs For DSCSA Mixed Period And Beyond
As enforcement takes root, readers at pharmaceutical manufacturers could be subject to inquiries/audits from both FDA and state boards of pharmacy regarding their DSCSA systems and processes.
In an August guest column, PDG Executive Director Eric Marshall alerted us to the mixed period coming up following the FDA’s complementary announcements:
- two-year extension for small dispensers and
- urging noncompliant trading partners to request individual WEEs.
Marshall reflected on FDA’s effort to maintain a delicate balance to limit disruptions while keeping pressure on the industry to continue its progress toward interoperable traceability. As he predicted, it would not be surprising to see FDA flooded with WEEs.
We all knew from the beginning that individual WEERs were not a simple solution and were fraught with compliance risks, since FDA couldn’t guarantee granting or denying waivers or exemptions by Nov. 27, given a large wave of requests. After receiving well over 300 WEERs and actively listening to industry stakeholders, FDA made this very pragmatic decision to ease the mixed period’s path forward, using a sweeping application of exemptions to the select hard-working, eligible manufacturers, distributors, and dispensers that are leading the efforts to exchange data effectively and efficiently under EDDS interoperability.
Management of the DSCSA’s complexity will fall most heavily on the good guys, those organizations that have done the long, hard work to ensure their internal systems and processes are best prepared for interoperable EDDS traceability. At a stabilized, steady state, trading partners must be exchanging and managing data for those tens of billions of individual drug package sales and purchases, and that interoperability must extend from highly sophisticated, multinational corporations to mom-and-pop pharmacies with limited resources and a plateful of competing demands. Data integrity and exceptions-handling efforts are particularly critical now.
Manufacturers’ cross-functional DSCSA supply chain, regulatory, and quality teams should be working with the HDA Exceptions Handling Guidelines and relevant PDG Blueprint guidance to ensure systems and processes (SOPs) are audit-ready, including verification and tracing for suspect or illegitimate product investigations.
See Appendix A, HDA DSCSA Exceptions Handling Guidelines & PDG Root Causes.
Is Your DSCSA Team Feeling Like A Le Mans Race Pit Crew?
Who can forget Matt Damon and Christian Bale’s characters in Ford vs. Ferrari engaging in the grueling Le Mans race?
Do you remember seeing the, “Goddamn NASCAR crew” scene with the specialized NASCAR pit crew taking care of business behind the racing stars?
The DSCSA teams at both pharma and generics manufacturers must feel like that pit crew, needing to perform behind the scenes like Le Mans or NASCAR teams, in this case keeping the Big Three wholesalers fueled up and humming in a fast-turnaround race that is pharmaceutical distribution.
If a manufacturer is not providing quality EPCIS data with its product, the racecar will never get out of the pit stop quarantine in November, and that manufacturer will be out of the race.
Don’t let valuable product get quarantined in a warehouse pit stop and never get back in the race. Keep racing with timely and high-quality DSCSA data attached to each product shipment!
Further Reading: Appendix A: HDA DSCSA Exceptions Handling Guidelines & PDG Root Causes; Appendix B: DSCSA Implementation: FDA & Industry Collaboration (2014-24)
About The Author:
 Carl Accettura is a 40-year veteran of FDA-regulated operations with diversified global experience in both large and small pharmaceutical manufacturers. He has made senior management-level contributions in the areas of global supply chain management and global drug development and commercialization, across numerous therapeutics and new product launches. He holds a B.S. degree in mechanical engineering from Cornell University, an M.Sc. degree in mechanical engineering from the University of Illinois, and an MBA from New York University.
Carl Accettura is a 40-year veteran of FDA-regulated operations with diversified global experience in both large and small pharmaceutical manufacturers. He has made senior management-level contributions in the areas of global supply chain management and global drug development and commercialization, across numerous therapeutics and new product launches. He holds a B.S. degree in mechanical engineering from Cornell University, an M.Sc. degree in mechanical engineering from the University of Illinois, and an MBA from New York University.