Trends And Benefits Of Lean Manufacturing In Pharmaceutical Injectable Facilities
By Ashok Kumar Dasari, Viatris BLR India

The pharmaceutical injectable manufacturing sector is experiencing unprecedented transformation through lean manufacturing adoption, delivering quantifiable operational improvements while maintaining stringent quality standards. This analysis examines current implementation trends, operational advantages, productivity impacts, and quality integration strategies based on real-world performance data from leading pharmaceutical organizations.
Current Implementation Trends Driving Industry Evolution
1. Digital Lean Integration: The Technology-Enabled Transformation
Modern pharmaceutical injectable facilities are pioneering sophisticated digital lean implementations that integrate advanced technologies with traditional lean principles. This convergence represents a fundamental shift from reactive to predictive manufacturing paradigms.
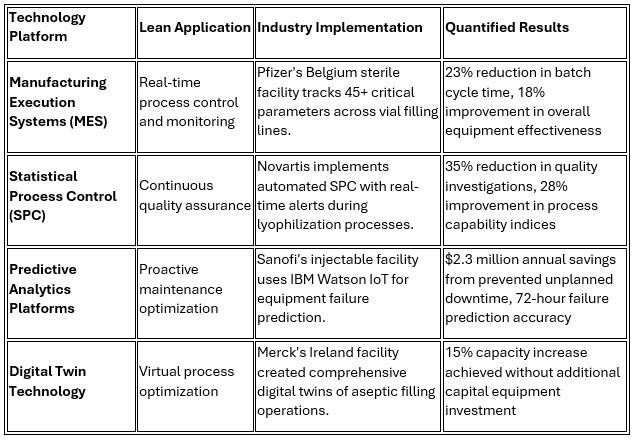
The integration of these technologies with lean principles creates unprecedented visibility into manufacturing processes while enabling real-time optimization. Roche's implementation of AI-powered quality prediction demonstrates the potential, with its system accurately predicting batch quality outcomes 24 hours before completion, allowing proactive adjustments that have improved first-pass yield rates by 12%.
2. Risk-Based Lean Methodologies: Regulatory Alignment
Contemporary lean implementations emphasize sophisticated risk-based approaches that align with regulatory expectations while delivering operational improvements. This methodology ensures compliance with GMP while maximizing efficiency gains.
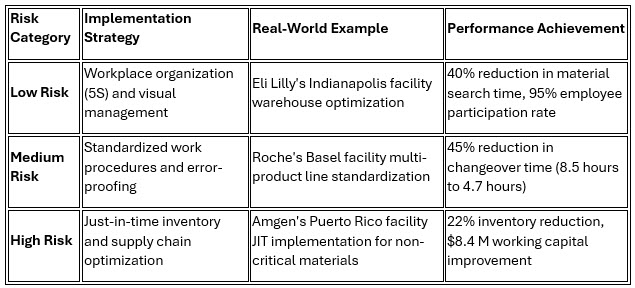
Johnson & Johnson's risk-based approach at its Leiden facility exemplifies this strategy, achieving 85% overall equipment effectiveness (OEE) on lyophilizer lines while maintaining zero regulatory observations during its most recent FDA inspection.
Operational Advantages: Quantified Performance Improvements
1. Enhanced Operational Efficiency Through Waste Elimination
Lean manufacturing principles demonstrate substantial capability for reducing manufacturing cycle times and eliminating non-value-added activities. Value stream mapping initiatives consistently achieve significant improvements across multiple performance dimensions.
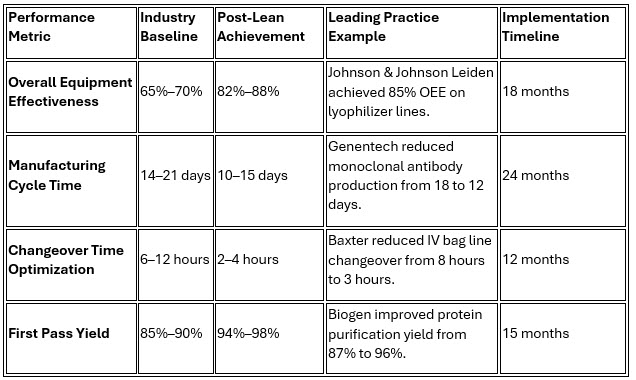
Genentech's transformation represents a benchmark implementation, where its systematic approach to waste elimination in monoclonal antibody production resulted in a 33% cycle time reduction while simultaneously improving quality metrics. This success demonstrates that efficiency and quality are complementary rather than competing objectives.
2. Resource Utilization Optimization
Standardized work procedures and cellular manufacturing principles significantly enhance equipment utilization rates while minimizing unplanned downtime. This optimization proves particularly valuable in injectable facilities where cleanroom infrastructure represents substantial capital investments.
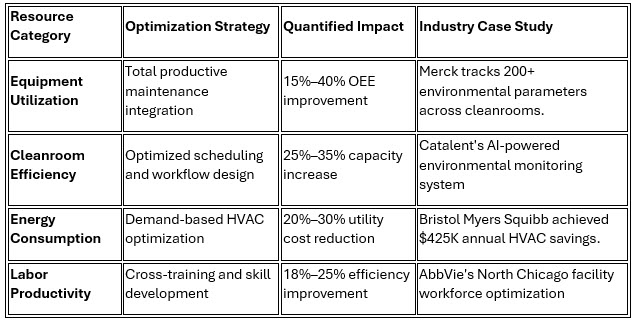
Catalent's innovative approach demonstrates the potential for resource optimization, where their AI-powered environmental monitoring system not only ensures sterility compliance but also optimizes energy consumption, resulting in $600K annual savings while improving process reliability.
3. Inventory Management and Working Capital Optimization
Just-in-time principles, when strategically adapted for pharmaceutical manufacturing requirements, generate substantial working capital improvements while reducing material-related risks.
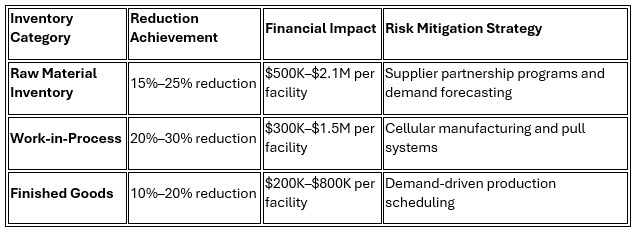
Gilead's success story illustrates optimal inventory management, where its systematic approach reduced API inventory by $1.8 million while maintaining 99.8% service levels through sophisticated demand forecasting and supplier collaboration programs.
Impact On Productivity And Management Paradigms
1. Productivity Transformation Through Systematic Improvement
Organizations implementing lean principles achieve dramatic productivity improvements across multiple dimensions, fundamentally transforming their operational capabilities and competitive positioning.
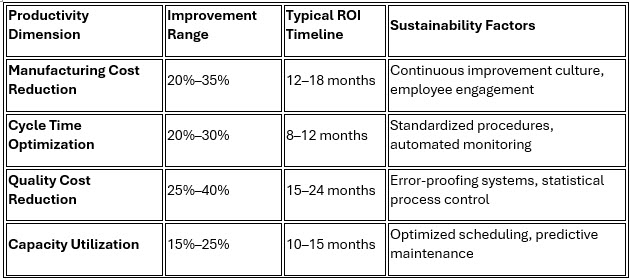
Pfizer's comprehensive transformation demonstrates the potential for productivity excellence, where its Puurs facility achieved a 24% manufacturing cost reduction (from $2.45 to $1.85 per unit) while simultaneously improving quality performance and employee satisfaction.
2. Management Philosophy Evolution
Lean implementation necessitates fundamental shifts in management philosophy from traditional command-and-control structures toward servant leadership methodologies that empower frontline personnel and foster continuous improvement cultures.
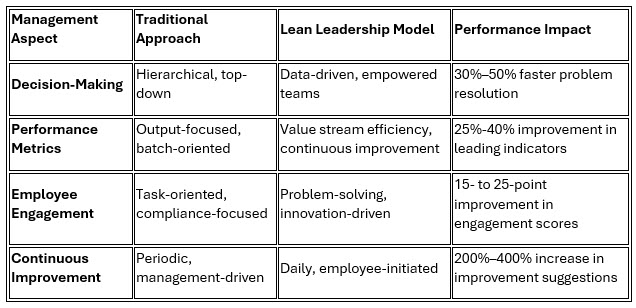
GSK's Singapore facility exemplifies successful management transformation, achieving 89% employee buy-in through a bottom-up approach that empowered operators to identify and implement improvements, resulting in over 300 implemented suggestions in the first year.
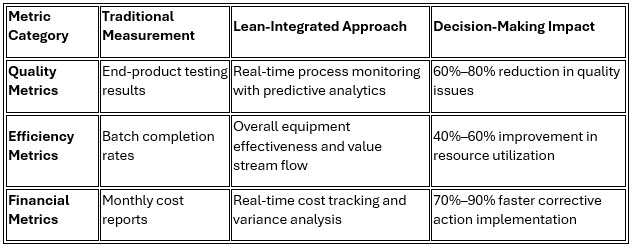
3. Metrics-Driven Decision-Making
Lean implementation introduces sophisticated performance measurement systems that provide real-time visibility into operational performance while enabling proactive decision-making.
Quality Attributes Integration: Excellence Through Systematic Approach
1. Positive Quality Impact Mechanisms
Lean manufacturing enhances quality outcomes through multiple interconnected mechanisms that address root causes of variation while building robust quality systems.
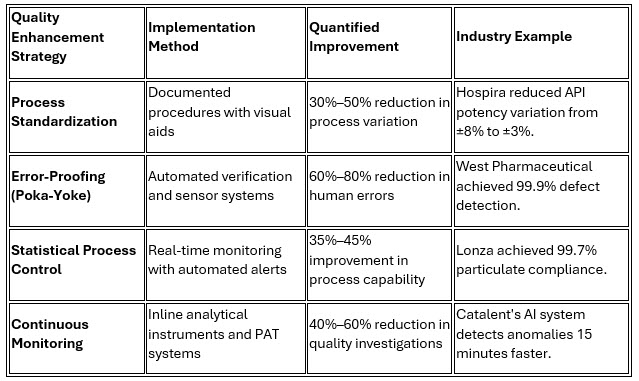
Lonza's comprehensive approach demonstrates quality excellence through systematic integration of lean principles with advanced quality systems, achieving 99.7% particulate compliance while reducing quality investigation time by 45%.
2. Critical Quality Attribute Optimization
Lean implementation enables enhanced monitoring and control of critical quality attributes through integrated systems that provide real-time visibility and proactive management capabilities.
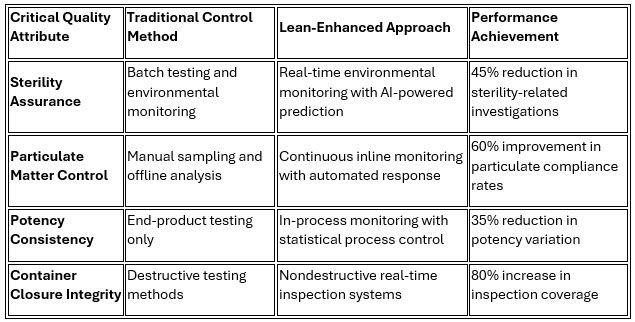
Roche's implementation of predictive quality systems exemplifies the potential for CQA optimization, where its AI-powered approach predicts batch quality outcomes 24 hours before completion with 94% accuracy, enabling proactive adjustments that have improved overall product quality by 18%.
3. Regulatory Compliance Enhancement
Lean principles, when properly implemented, strengthen rather than compromise regulatory compliance by creating more robust, transparent, and efficient quality systems.
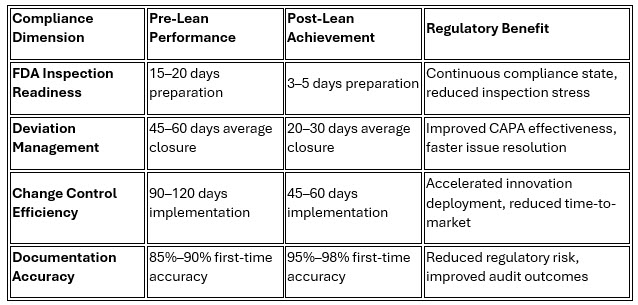
Regeneron's success in maintaining zero FDA observations during its lean transformation demonstrates that operational efficiency and regulatory compliance are mutually reinforcing when properly integrated.
Financial Performance And Return On Investment
1. Comprehensive Cost Impact Analysis
Lean implementation generates substantial financial benefits across multiple cost categories, creating compelling business cases for transformation initiatives.
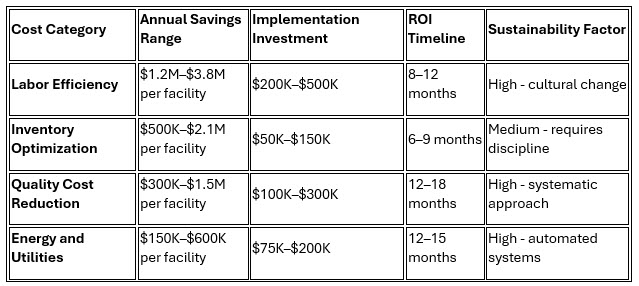
AbbVie's North Chicago facility achieved $2.4 million in annual labor efficiency savings through systematic workforce optimization and cross-training programs, with payback achieved in just 10 months.
2. Performance Scalability by Facility Size
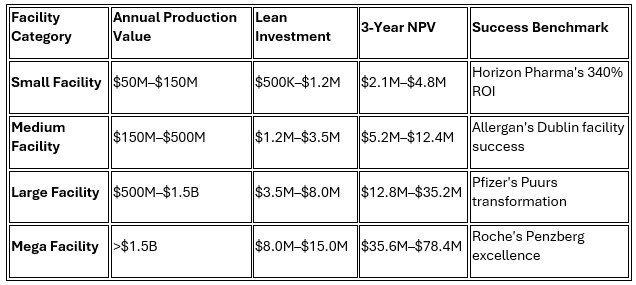
Future Opportunities And Strategic Implications: Emerging Technology Integration
The convergence of lean manufacturing with Industry 4.0 technologies creates unprecedented opportunities for pharmaceutical injectable facilities to achieve new levels of operational excellence.
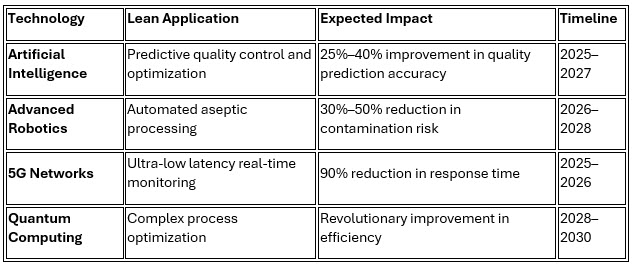
Novartis' pioneering work with collaborative robots in sterile environments demonstrates the potential for advanced automation integration, achieving 35% improvement in processing consistency while maintaining full regulatory compliance.
Strategic Recommendation: Realize Advantages Through Immediate Opportunity Assessment
Organizations should conduct comprehensive assessments to identify high-impact opportunities for lean implementation that align with their strategic objectives and regulatory requirements.
Key Assessment Areas:
- Current state value stream analysis with quantified waste identification
- Regulatory risk evaluation and compliance gap analysis
- Cultural readiness assessment and change management requirements
- Financial modeling with realistic ROI projections and risk scenarios
Technology Integration Road Map
Successful lean transformation requires strategic technology integration that enhances rather than complicates operational processes.
Priority Implementation Sequence:
- Foundation Systems (0-6 months): MES, SPC, basic automation
- Advanced Analytics (6-18 months): Predictive systems, AI integration
- Autonomous Operations (18-36 months): Advanced robotics, full integration
Performance Measurement Framework
Comprehensive measurement systems ensure sustainable value realization while maintaining regulatory compliance and quality standards.
Essential Metrics:
- Operational efficiency indicators (OEE, cycle time, yield)
- Quality performance measures (deviations, CAPAs, compliance)
- Financial impact tracking (cost reduction, ROI, working capital)
- Employee engagement and cultural transformation indicators
Conclusion: The Competitive Imperative
The evidence overwhelmingly demonstrates that lean manufacturing implementation in pharmaceutical injectable facilities delivers transformational advantages that extend far beyond cost reduction. Organizations achieving excellence in lean implementation gain sustainable competitive advantages through enhanced operational efficiency, improved quality performance, and empowered workforce cultures.
The pharmaceutical industry's evolution toward personalized medicine, increased regulatory scrutiny, and intensified cost pressures make lean manufacturing not merely advantageous but essential for long-term success. Companies that embrace comprehensive lean transformation will define the future of pharmaceutical manufacturing excellence while fulfilling their fundamental mission of delivering lifesaving medications to patients worldwide.
The time for incremental improvement has passed. The future belongs to organizations that commit to operational excellence through strategic lean implementation, supported by advanced technology integration and unwavering focus on quality outcomes.
Disclaimer: The information provided in this document is intended for general informational purposes only. The details and company names mentioned herein have been sourced from various social media platforms and internet resources. While every effort has been made to ensure the accuracy of the information, the author does not guarantee its completeness or reliability. The author shall not be held liable for any errors, omissions, or any outcomes related to the use of this information.
About The Author:
 Ashok Kumar Dasari is a life science professional at Viatris BLR India. He is a seasoned biopharmaceutical professional with extensive expertise in pharmaceutical practices, aseptic operations, regulatory compliance, and total quality management systems. With a career that spans both local and international landscapes, he has consistently worked to address the evolving challenges of the modern pharmaceutical industry. Dedicated to lifelong learning and knowledge sharing, he remains committed to writing on novel topics in the pharmaceutical domain and beyond, offering narratives that inform, inspire, and innovate.
Ashok Kumar Dasari is a life science professional at Viatris BLR India. He is a seasoned biopharmaceutical professional with extensive expertise in pharmaceutical practices, aseptic operations, regulatory compliance, and total quality management systems. With a career that spans both local and international landscapes, he has consistently worked to address the evolving challenges of the modern pharmaceutical industry. Dedicated to lifelong learning and knowledge sharing, he remains committed to writing on novel topics in the pharmaceutical domain and beyond, offering narratives that inform, inspire, and innovate.
