The Benefits Of Using GenAI In Drug Discovery And Preclinical Development
By Adam Berman and Kevin L. Anderson Jr., EY-Parthenon

The emergence of generative artificial intelligence (GenAI) has the potential to reform early-stage drug discovery and development. Large language models are being harnessed to create novel molecules tailored for specific properties, positioning them as potential drug candidates while also revolutionizing various aspects of the drug development process.
This innovative methodology promises to potentially mitigate formidable costs and time constraints traditionally associated with drug discovery. It could also unveil previously overlooked therapeutic possibilities. To achieve these benefits, though, pharmaceutical company leaders need to make sure they have a sound strategy to implement and support the technology, as well as to manage the massive organizational change it will involve.
How GenAI Can Address Multiple Drug Discovery Issues
The profound impact of AI on the pharmaceutical industry is evident in the rapid adoption of digital transformation. Over the past several years, a surge of interest in AI technology has emerged. This has been supported by the proliferation of GenAI focused startups and substantial investment and increased collaborations between pharmaceutical companies and AI vendors. A particular emphasis has been the integration of GenAI solutions.
The use of GenAI holds great potential for a drug discovery process known to take up to a decade or longer, with an average cost of $1 billion to $2 billion per therapy that makes it to the market. GenAI has the potential to expedite and reduce the cost of every step of the drug discovery and early-stage development process, where only 10% of candidate molecules advance to clinical trials (Figures 1 and 2).
Industry experts anticipate that GenAI will have a noticeable impact across various elements of drug discovery and development, resulting in cost and timeline reductions as adoption becomes more prevalent and models become more optimized. The potential uses begin in key steps in drug discovery, such as:
- Target Identification
Deep learning algorithms can be used in virtual screening to predict and prioritize potential drug-target interactions, facilitating rapid identification of attractive compounds and efficient screening of chemical databases. At the same time, employing generative models and thorough data analysis can help companies discover existing drugs suitable for repurposing into new therapeutic uses.
- Target Validation
De novo drug design, or the generation of entirely new molecules with specified properties, can be aided by generative models to comprehensively explore the vast chemical space and design custom compounds tailored to a specific target or disease. Many of these compounds may have been challenging or time-consuming to discover through traditional methods. More efficient drug synthesis and testing may also be possible, given focused resources on the most promising candidates, which reduces the likelihood of false positives and streamlines the validation process.
- Hit Generation
Deep learning techniques can predict protein drug interactions, binding affinity, and undesirable side effects to accelerate identification of compounds with therapeutic effects. GenAI also can help generate novel chemical structures and predict viable synthetic routes to ensure feasibility of chemical synthesis.
- Lead Optimization
The discovery process can be accelerated by leveraging advanced algorithms and data analysis to identify promising candidates with the ideal therapeutic properties.
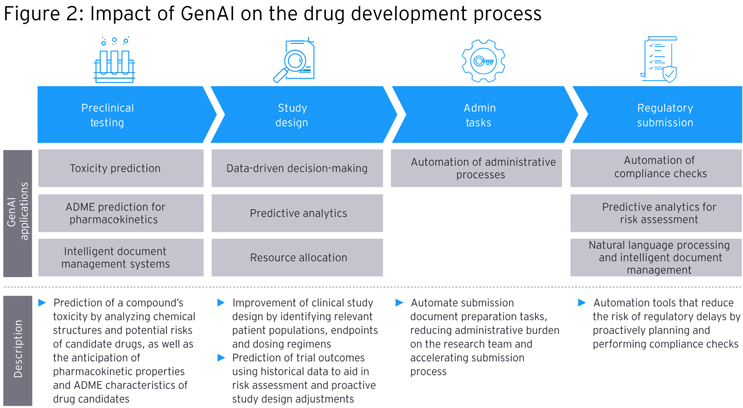
Use Of GenAI In Key Drug Development Steps
Drug development also can be aided by GenAI in several ways:
- Preclinical Testing
GenAI can help predict the toxicity of a drug compound by analyzing chemical structures and potential risks associated with candidate therapies. It also can help forecast pharmacokinetic properties and ADME characteristics of drug candidates, which can inform how a given drug will act against its target as well as how safe it is for the patient.
- Study Design
GenAI can enrich data-driven decision-making, such as by helping to improve clinical study design by identifying the most relevant patient populations, endpoints, and dosing regimens. It also can predict clinical trial outcomes using historical data to aid in risk assessment and proactive study design adjustments to increase the likelihood of success and reduce costly failures.
- Automation Of Administrative Processes
GenAI can help optimize resource allocation by predicting which studies are most likely to succeed and have the greatest impact. Additionally, AI technology also can automate administrative tasks such as patient recruitment, data entry, and regulatory document management, saving time and minimizing errors.
- Regulatory Submissions
A combination of both GenAI and more generic AI technology can aid in the integration and analysis of diverse data sources, speeding up development of regulatory submissions and reducing errors. GenAI can be used to automate compliance checks, reducing the risk of regulatory delays by proactively performing checks against guidelines to ensure that submissions adhere to all requirements.
Separately, GenAI’s predictive analytics can be used to assess potential risks associated with regulatory submissions to help companies make informed decisions and mitigate delays.
Lastly, natural language processing (NLP), a form of AI, can be used to expedite document creation and validation to enhance overall quality and accuracy in regulatory submissions.
Overall, an EY-Parthenon survey of large, medium, and small biopharma sponsors indicates that GenAI is expected to have the largest impact on compound screening and prioritization, target identification and validation, as well as prediction of drug-drug interactions. Lower impact is expected to be seen in biomarker discovery and clinical trial design.
Expected Impact Of GenAI On Cost Reduction
EY teams interviewed 15 senior research and development decision makers at biopharmaceutical and biotech companies to get a sense of the potential for GenAI. As the technology is adopted over time, the interviewees agreed that future cost reductions will increase significantly for all phases of the drug development value chain (Figure 3). However, the extent of these cost savings might vary significantly across different phases due to the unique advantages and challenges presented by each step.
Source: EY-Parthenon interviews with senior executives.
Bar chart indicating the average forecast for cost reductions from GenAI in different areas of drug discovery and development.
Interviewees said that in the next three to five years, cost reductions from GenAI across all phases will range from 15%-22%. In five to seven years, cost savings are expected to increase to a range of 22%-33%. Once peak adoption of GenAI is reached, the expected range of cost savings was forecast to be 44%-67%. Primary opportunities for cost reduction, and the average estimate of the decision makers interviewed, include:
- Target identification (67% reduction at peak adoption): Target identification was cited as the greatest cost reduction area as GenAI powered virtual screening is expected to be rapidly adopted.
- Target validation (66% reduction at peak adoption): Many experts believe GenAI will increase the speed of target validation through virtual drug design and chemical exploration, reducing costs significantly once fully adopted.
- Lead optimization (63% reduction at peak adoption): Lead optimization is expected to experience substantial cost reduction due to GenAI, potentially aided by GenAI's ability to optimize hit compounds rapidly, resulting in a shorter and more efficient lead optimization process. At the same time, the precision of AI in refining chemical structures and predicting pharmacological properties can contribute to this cost-saving potential.
- Study design (62% reduction at peak adoption): GenAI is poised to significantly reduce the cost of study design by leveraging data-driven decision-making. AI's capacity to analyze extensive data sets and identify optimal study parameters might streamline the process, reducing resource allocation inefficiencies. This cost reduction is particularly promising for resource-intensive clinical trials.
Secondary Cost Reduction Opportunities Using GenAI
Beyond the primary opportunities for cost reduction are other areas that may be streamlined, though the cost benefits are less sure:
- Administrative tasks (56% reduction at peak adoption): Administrative tasks are essential but not inherently experimental. GenAI can help automate and streamline document management and compliance checks, but the cost savings might not be as pronounced as in other research-oriented phases.
- Hit generation (56% reduction at peak adoption): Hit generation is expected to benefit significantly from GenAI due to its ability to predict interactions and design novel compounds. However, the cost reduction might not be as high as in lead optimization because hit generation often involves more preliminary and exploratory work.
- Regulatory submission (54% reduction at peak adoption): Regulatory submission is expected to benefit substantially from GenAI. GenAI's ability to automate tasks can enhance the efficiency of this phase, reducing costs associated with extensive regulatory compliance.
- Preclinical testing (44% reduction at peak adoption): Although GenAI can predict toxicity and pharmacokinetics efficiently, preclinical testing encompasses a wide range of experiments and assessments, making it less susceptible to dramatic cost reductions. The complexity of testing might limit the extent of cost savings.
Considerations For How Organizations Can Utilize GenAI In Drug Discovery
Given the number and diversity of therapeutic areas and indications being targeted by large pharma companies, as well as the number of resources being deployed, pharmaceutical companies are willing to make larger investments in technologies such as GenAI to get their products to market fastest. EY professionals worked with a large pharmaceutical company invested in enterprise-wide GenAI capabilities that most notably reduced the total time from novel target identification to a drug in clinical trials from months or years to weeks.
According to a former principal scientist at a large biopharma organization, “Large biopharma companies already have experience implementing AI and ML models, throughout their organization, positioning them to continue investing in and adopting GenAI capabilities.”
Small to midsized biopharma companies, on the other hand, typically lack internal resources and funding to implement GenAI and train their talent on its capabilities, which limit its adoption in this market segment. Nonetheless, startup companies designed with the intention of implementing GenAI in drug discovery have been successful. For example, a drug discovery company combining large-scale data with machine learning is leveraging its GenAI models to accelerate the discovery of novel small molecules.
Pharmaceutical companies’ desire to use GenAI could also be an opportunity for contract development and manufacturing organizations (CDMOs), contract research organizations (CROs), and software companies, since smaller biopharma companies usually do not have internal human resources to develop or optimize their own GenAI algorithm or software. As a result, CDMOs and CROs are likely to invest in GenAI capabilities over the next three to five years, so they can improve and differentiate their drug discovery offerings, such as virtual screening, to continue to drive business with biopharmaceutical companies of various sizes.
Developing A Strategy To Create An GenAI-supported Organization
The benefits of using GenAI in drug development are clear: the technology has the potential to broaden the diversity of treatments, reduce development costs, increase drug approval rates, and get treatments to patients faster. But not every organization has the financial wherewithal or internal knowledge and capabilities to proceed with this nascent technology.
In order to develop a successful GenAI strategy, organizations need to survey the software and application landscape to determine what is possible and what would best support their long-term growth goals. Then you can determine whether it is more beneficial to develop your own GenAI applications, to buy the technology from a vendor, or to partner with a third party. Once you have decided to implement GenAI in drug discovery, you also will need to deploy a change management plan that supports the long-term growth of the organization and is in line with the organization’s culture. Lastly, it’s important to take steps to ensure effective governance over any GenAI model that is designed and implemented, establishing clear policies and guidelines, maintaining stringent data quality and management practices, executing regular audits and assessments of the GenAI system(s), and fostering an organizational culture that understands and respects the ethical implications of generative AI use.
The views reflected in this article are the views of the authors and do not necessarily reflect the views of the global EY organization or its member firms.
 About The Authors:
About The Authors:
Adam Berman is a senior director in the EY-Parthenon Life Sciences Strategy practice with over 28 years of industry and consulting R&D specific experience advising senior executives of life sciences organizations. In addition to his time at EY-Parthenon, he has worked extensively within the R&D function for a number of organizations. Berman spends his time serving biopharmaceutical companies, clinical research organizations, pharmaceutical service organizations, and others in the R&D ecosystem.

Kevin L. Anderson Jr. is a manager in the EY-Parthenon Life Sciences Strategy practice with over six years of experience advising senior executives of life sciences organizations. He is a physician by training with additional training in R&D and clinical research. Anderson spends his time serving biopharmaceutical companies, clinical research organizations, retail pharmacies, and others in the R&D ecosystem.