Reorganization Of The Office Of New Drugs With Corresponding Changes To The Office Of Translational Sciences And The Office Of Pharmaceutical Quality
In 2017, FDA’s Center for Drug Evaluation and Research (CDER) embarked on an initiative to modernize the New Drugs Regulatory Program. For more information on this initiative, please visit Modernizing FDA’s New Drugs Regulatory Program. (https://www.fda.gov/drugs/regulatory-science-research-and-education/modernizing-fdas-new-drugs-regulatory-program)
One critical component of the Modernization includes a reorganization of the New Drugs Regulatory Program. The reorganization of the New Drugs Regulatory Program requires restructuring of the Office of New Drugs (OND) and corresponding changes in the Office of Translational Sciences (OTS) and Office of Pharmaceutical Quality (OPQ). The approved changes in OND will create offices that align interrelated disease areas, and divisions with clearer and more focused areas of expertise. The changes increase the number of OND offices that oversee our review divisions from six to eight—and increases the number of OND clinical divisions from our current 19 divisions to 27, plus six non-clinical review divisions. In addition to enabling greater efficiency, these envisioned changes will help us to better understand the diseases intended to be treated by the drugs we evaluate for approval – another way we aim to enhance our scientific leadership. OND will also create cross-functional support offices of New Drug Policy, Drug Evaluation Sciences, Regulatory Operations, Operations, and Administrative Operations.
Office of New Drugs (https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/office-new-drugs)
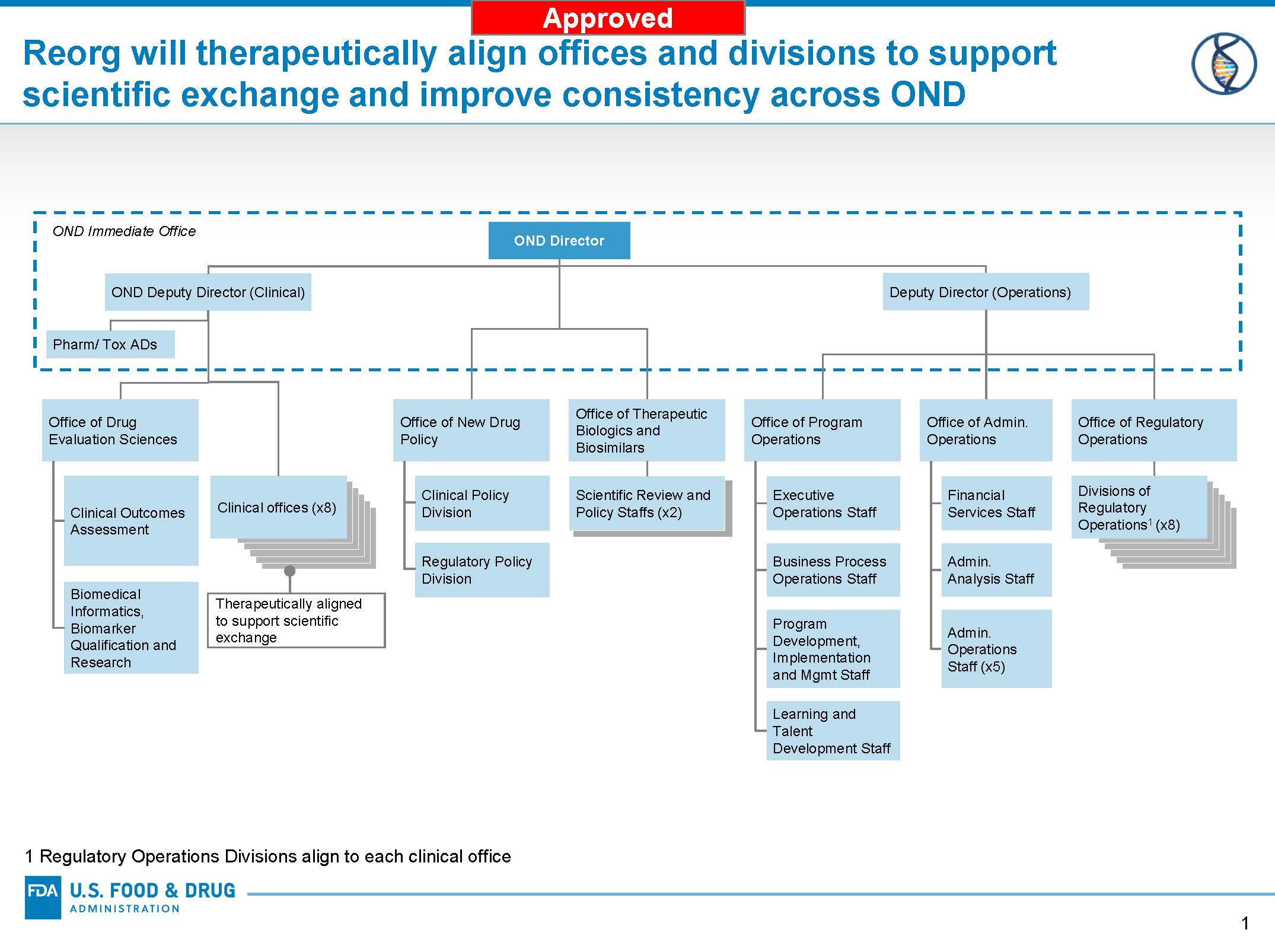
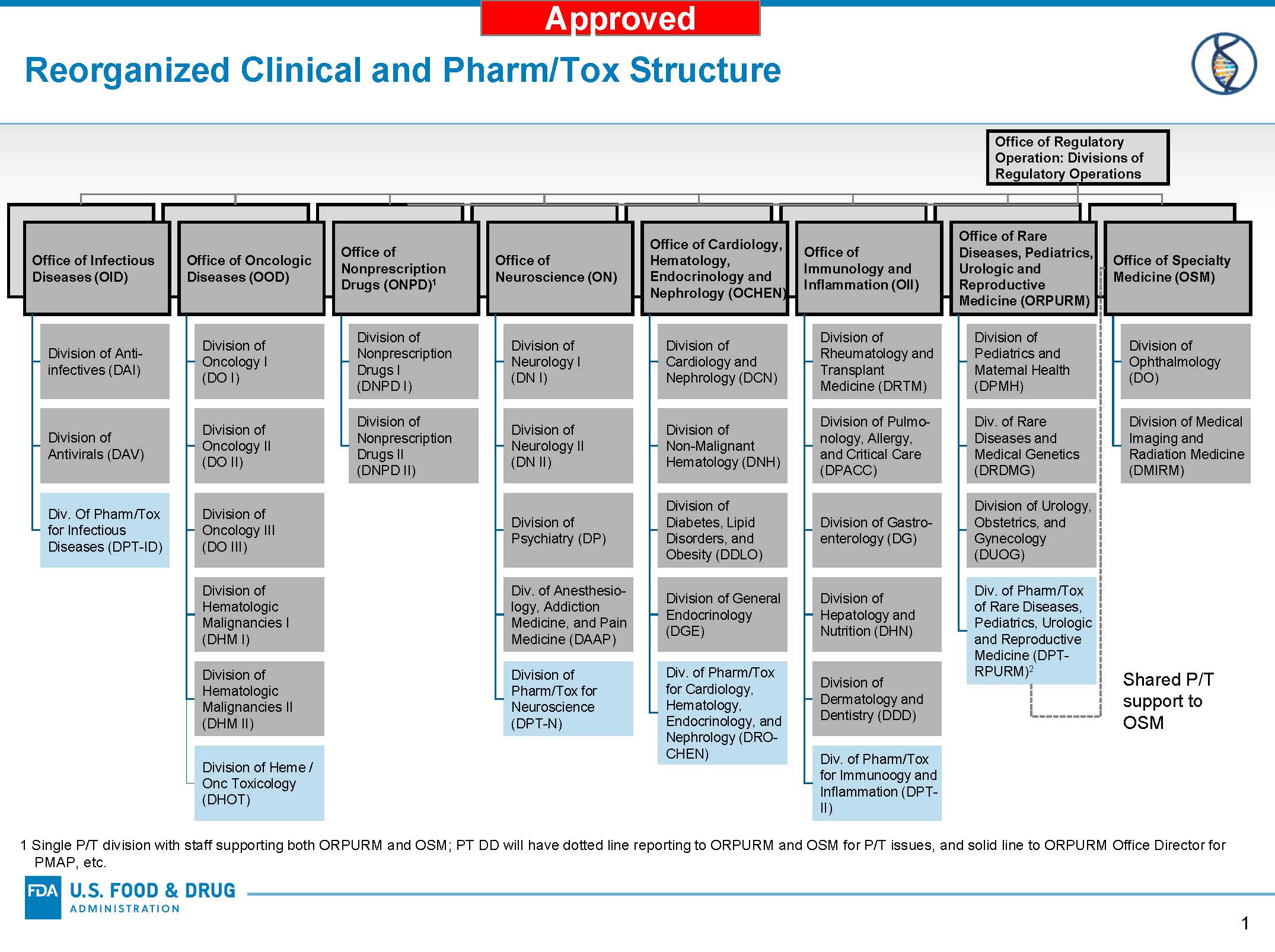
Check back regularly for more information on OND’s implementation of the reorganization.
Changes within other New Drugs Regulatory Program offices
- The Office of Translational Sciences (OTS) will align to support the clinical structure within OND. OTS’ overarching rationale for the reorganization is to achieve minimal disruption and enhance OND alignment. The Office of Biostatistics (OB) will reorganize from 8 to 9 therapeutically aligned divisions. The Office of Clinical Pharmacology, will reorganize from current 5 to 7 therapeutically aligned divisions. In both offices, cross-cutting functions will be consolidated for better review quality and process efficiency. The OTS Immediate Office will establish an Office of Administrative Operations.
- The Office of Pharmaceutical Quality (OPQ): is changing their structure to better align within OPQ, with the New Drug Regulatory Program modernization, and with other groups in CDER. The modernization efforts created a window of opportunity to re-vision and reorganize some parts of OPQ while maintaining its core work functions. OPQ is establishing a new OPQ Office of Administrative Operations, renaming two existing OPQ offices, and creating seven new divisions across OPQ.
- The Office of Surveillance and Epidemiology (OSE) will realign within teams and does not require reorganization.
Who to contact for more information about the reorganization of OND or the NDRP Modernization?
For more information on the reorganization of OND or the NDRP Modernization, please email druginfo@fda.hhs.gov.
Reorganization Questions and Answers
- I have questions about the reorganization. Where can I get additional information?
We will post information and updates on the reorganization on the Reorganization of the Office of New Drugs with Corresponding Changes to the Office of Translational Sciences and the Office of Pharmaceutical Quality website. If you have specific questions, please reach out to druginfo@fda.hhs.gov. - How will this affect processes and communications with industry? Will FDA contacts change?
We are conducting assessments of risk and preparing across our operations to ensure that our interactions with industry are minimally affected. Our communications will increase during the reorganization process to assure industry stays up to date with any changes to responsible organizations of their applications (i.e., INDs and NDA/BLAs), and any changes to points of contact. A website with information on the reorganization, the implementation plan, changes to FDA points of contact, application assignments, and additional resources will be launched coinciding with the reorganization’s start. We encourage industry to review the website carefully and check back throughout the reorganization process. - Will my NDA or BLA applications that are currently in review by OND divisions be affected? If so, how?
Review teams will not change. However, the responsible division and signatory responsible for NDA and BLA applications under review may change – this will be determined by several factors, including how far along the review of the application is at the time of the reorganization of that division. If there is a change in responsible organization or point of contact for applications currently under review, then Sponsors will be notified. Sponsors can also see if their applications are affected by viewing the list of application (INDs and NDA/BLAs) numbers and the associated point person on the NDRP Modernization Reorganization webpage. - As a result of the reorganization, will there be changes to CDER Manual of Policies and Procedures (MAPPs) as well as guidance documents related to INDs and NDA/BLA marketing applications?
CDER Manual of Policies and Procedures and guidance documents related to INDs and NDA/BLA marketing applications may be updated as a result of the reorganization to reflect updated organizational titles and processes. These administrative updates are not expected until the reorganization is complete. Updated documents and policies and procedures will be posted on the FDA website. - Will there be changes in the way CDER communicates and interacts with sponsors?
The reorganization will not directly change the way CDER communicates and interacts with sponsors. However, we believe that these changes to the organizational structure of the New Drugs Regulatory Program will enable CDER to communicate in a more efficient and effective way with Sponsors. - When will CDER update their webpages to reflect these organizational changes?
These updates will be completed over the course of the reorganization, with updates to websites coinciding with the reorganization of structures.
Additional information on the goals of modernization
- Dr. Woodcock FDA Voices blog – FDA Proposes Process Modernization to Support New Drug Development
- Statement from FDA Commissioner Scott Gottlieb, M.D., on proposed modernization of FDA’s drug review office
- Dr. Woodcock video - State of CDER 2019 (:41-2:30)External Link Disclaimer
- Dr. Woodcock's presentation at FDLI Annual Conference: Exploring Advanced Topics in Food and Drug Law
Return to Modernizing FDA's New Drugs Regulatory Program
Source: U.S.Food and Drug Administration
