Pharmaceutical Quality Management System (QMS) Software
Managing documents, training, and business processes are nightmares shared by all pharmaceutical and biotech companies and MasterControl provides pharmaceutical quality management software systems to take them out from that nightmare. MasterControl's QMS software exchanges the nightmares for a dream of a solution that enables pharma companies get their products to market faster and improve compliance at the same time. MasterControl pharma QMS software has been specifically designed to help manage all documents and automate regulatory-related processes and training in a single platform.
Who Can Benefit from Pharmaceutical QMS Software?
- R&D Managers (Regulatory, Clinical, and Pre-Clinical):
Can use MasterControl TotalPharma, a pharmaceutical quality management software system, to more efficiently organize, search, and take inventory of various study documents within the centralized, secure MasterControl system. With MasterControl's automated routing and approval functionality, managers can more easily oversee project teams composed of resources from multiple departments, even when those teams are working on multiple projects at the same time. MasterControl's pharmaceutical quality management software also ensures that correspondence from regulatory agencies, CROs, and suppliers is linked to the appropriate documentation.
- R&D Document Authors:
A pharmaceutical quality management software system provides author documents easily from compliant templates and author do not have to worry about reworking them to match the templates. Users of Word 2007 can create, revise, and redline documents without leaving Word using the new MasterControl toolbar. With MasterControl, PDFs with content bookmarks can be automatically created for regulatory submissions as well as document control functions. Since MasterControl's pharmaceutical quality management system is a connected and complete system, authors won't have to constantly move back and forth between disparate systems (from e-mail to Word and then to the document management system, etc.) in order locate a document, revise it, and then send it on for review or approval - MasterControl allows all such actions to be taken within the same integrated system.
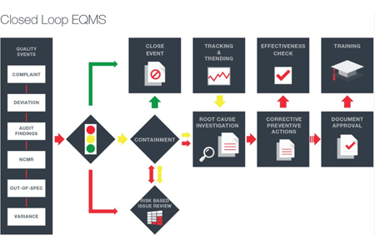
- Manufacturing/Operations:
Can easily track all specifications, deviations, and nonconformance's throughout the development cycle. MasterControl TotalPharma ensures that all appropriate personnel are trained on the most up-to-date SOPs and work instructions. The system also automates training tasks and allows training records to be located without difficulty in a centralized system.
- Clinical Personnel:
Our pharmaceutical quality management software can avoid paper filing backlogs that create a "black hole" of documents that are nearly impossible to find. Because the MasterControl system is electronic and automated, searching archives for trial documentation (protocols, IRB information, etc.) is simple. Electronic copies of documents, e-mails, CVs, etc., from study sites are readily accessible in a single, centralized system.
- Management:
MasterControl TotalPharma can provide a comprehensive solution for any life science organization regardless of existing in-house capabilities. Whether the organization simply needs training on the system's functionality or requires assistance configuring the system to specific needs, MasterControl's pharmaceutical quality management software systems can be packaged with any level of service and support the organization requires. An organization requiring expert knowledge can have MasterControl's skilled advisory team analyze the circumstances and develop and clearly map out those requirements.
