Phages As A Pharmaceutical: New EMA Guidance On Antimicrobial Drug Development
By Tim Sandle, Ph.D.
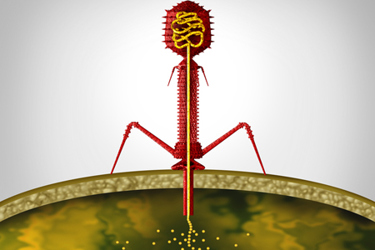
Bacteriophages are viruses that infect (through injection of the viral genome into the bacterial cell cytoplasm) and can kill bacteria. Interest in the use of phage therapy began in the 1930s; it was then abandoned due to the development of antibiotics. Due to the rise in antimicrobial resistance, interest in bacteriophages as a means to target pathogenic bacteria has increased in recent years. With the corresponding drug development comes the necessity of a regulatory framework.
The European Medicines Agency (EMA) has issued a draft document, “Guideline on quality aspects of phage therapy medicinal products,”1 outlining the regulatory expectations for quality documentation of bacteriophage active substances and finished products for human use. This relates to the documentation required for new marketing authorization applications. It addresses specific aspects regarding the manufacture, control of materials, characterization, specifications, analytical control, reference standards, and stability of bacteriophage active substances.
Bacteriophages
There are thousands of bacteriophages in the environment, and they are widely distributed wherever bacterial hosts are found, like in the sea, the soil, and animals.2 Some estimates put the number of these viruses at around 1031. 3 From this, bacteriophages are clearly the most abundant biological entities on the planet.
The essentials of phage infection of bacteria (and hence of phage therapy) are:
- Bacteriophages kill bacteria by making them burst or lyse. This happens when the virus binds to the bacteria. A virus infects the bacteria by injecting its genes (either DNA or RNA).
- The phage virus reproduces inside the bacterium. This leads to 1,000 new viruses being formed in each bacterium.
- Following replication, the virus breaks open the bacteria, releasing the new bacteriophages.
- Bacteriophages can only multiply and grow inside a bacterium. Once all the bacteria are lysed, the phages stop multiplying.
- Like other viruses, phages can lie dormant until more bacteria are present.
Bacteriophages infect bacteria through the injection of the viral genome into the bacterial cell cytoplasm.4 It is this mechanism that some pharmaceutical researchers are keen to exploit.
Phage Therapy
Phage therapy refers to the therapeutic use of lytic bacteriophages for the treatment of pathogenic bacterial infections. A key advantage of phage therapy is that bacteriophages are far more specific than many of the antibiotics in clinical use. The active substance of the drug substance is manufactured from a selected host bacterium. Each bacterial cell is infected, and a process of controlled propagation is initiated to create the phage seed lot (from a single phage clone). Pharmaceutical manufacturers are required to demonstrate that the resultant phage population is genetically and phenotypically consistent.
Phage therapy medicinal products (PTMPs), as either single phages or combinations of phages, are a form of biological medicinal products and hence their development partly falls under existing regulatory requirements for biologics.5 However, there are characteristics of phages that make them different from other biological products, so specific guidance is required. These characteristics include:
- high specificity,
- self-propagation,
- potential for evolution, and
- the risk of horizontal gene transfer.
It is these core areas that the guidance is seeking to address.
What’s In The Proposed Guidance?
The draft guidance from the EMA applies only to virulent phages, either naturally occurring or those that have been chemically or genetically modified. This is an important distinction since, where genetic manipulation occurs, the phage falls under a gene therapy classification. This means that advanced therapy medicinal product (ATMP) requirements also apply, under EU legislation.
Phage Characterization
The first main requirement of the guidance is for each phage to be formally characterized. This requires:
- taxonomic classification,
- target bacteria for the phage to kill or inactivate,
- intended potency,
- phage particle size,
- phage genome type and size,
- detail of any notable genes present, and
- any genetic or chemical modifications performed to produce the phage.
Related information required to support a regulatory submission includes:
- bacterial cell bank and
- phage seed lot manufacturing, testing, and storage sites.
Manufacturing Process
The submission will need to include standard information about the manufacturing process:
- Intended batch scale and size (including any proposed range)
- Details of any planned blending of batches or sub-batches
- An overview of the manufacturing process (supported by flow diagrams)
- Details of mixing studies
- Detailed descriptions of each process step
Reviewers will be looking to see that the production process will yield a phage active substance of consistent quality and stability, as evidenced by process controls around each key step and relevant testing of intermediates (with details of the intended testing and acceptance criteria). Again, consistent with most biologic manufacturing, hold times should be defined with supporting data to show physicochemical, biological, and microbiological quality under each proposed storage condition.
Material Controls
As well as the standard requirements for material controls, the draft guidance focuses on two specific aspects.
Bacterial Cell Banks
The regulatory submission will require a description of the origin, history, and preparation method of the bacterial cells used for banking (with reference to European Pharmacopeia chapter 5.316 and ICH guideline Q5D7). The guidance refers to a recommendation for a two-tiered seed lot system. This means using a master seed lot and a working seed lot to minimize the number of passages (or subcultures) from the original source material. This ensures that unintended genetic drift is avoided.8
To demonstrate appropriate purity and consistency, the guidance requires the entire bacterial genome and plasmids of the master cell bank to be sequenced using next-generation sequencing supported by bioinformatic analysis. This should result in profiles for:
- Identity (from full genome sequencing). It is important to undertake work that encompasses a diverse range of bacteria, including multiple strains of the target species as well as closely related species, so that the characteristics of the selected host are noticeably clear.
- Purity (absence of detrimental phage particles) and a description of cell host proteins, so these can be detected as part of the intermediate manufacturing check for impurities.
- Cell viability.
- Phage sensitivity.
- Antibiotic susceptibility.
There is also a requirement to understand any potential detrimental factors, such as antibiotic resistance determinants or toxins.
Phage Seed Lots And The Use Of Phages In The Manufacturing Process
The phages used to generate a seed lot must be lytic, and the origin, history, and preparation of phage seed lots need to be documented. In the guidance, “lytic phage” refers to a type of bacteriophage that replicates and kills its host cell in a process referred to as the lytic cycle. Here, the phage takes over the bacterial cell's machinery to create new viral particles and then causes the cell to burst, releasing the new viruses to infect other cells.9 A second requirement is to understand the mediated transduction capability of the phage. This refers to the ability of some phages to package host bacterial DNA into their capsid alongside their own DNA and transfer it into a receiving cell,10 which is an undesirable function.
As with the characterization of the bacterial cell host, full genome sequencing is required along with the other requirements discussed above. In addition, there are some specific recommendations for the techniques required for the characterization:
- Phage structure by electron microscopy
- Phage phenotype by image analysis
- Phage genome characterization by nucleic acid sequencing
- Phage potency by plaque assay to determine the infectious phage titer range (as per draft European Pharmacopeia chapter 2.7.38, expected to be issued in 2026)11
It is also necessary to understand the likelihood or otherwise of phage aggregation (which can lead to a decrease in the infectious titer).
Other Requirements
The guidance sets out other requirements for the manufacture of a biologic, including the need for process validation; the requirement for end product sterility testing; stability trials; and the necessity to understand any impurities produced during the manufacturing process and to ensure mechanisms are in place for their detection, including pyrogens.
References
- EMA. Guideline on quality aspects of phage therapy medicinal products, EMA/CHMP/BWP/1/2024, Committee for Medicinal Products for Human Use, Netherlands
- Xue, C. and Goldenfeld, N. Coevolution Maintains Diversity in the Stochastic 'Kill the Winner' Model. Physical Review Letters, 2017 DOI: 10.1103/PhysRevLett. 119.268101
- Wommack KE, Colwell RR Virioplankton: viruses in aquatic ecosystems Microbiol. Mol. Biol. Rev. 2000; 64 (1): 69–114
- Keen EC Felix d’Herelle and our microbial future. Future Microbiol. 2012; 7(12): 1337-1339
- EMA Guideline on process validation for the manufacture of biotechnology-derived active substances and data to be provided in the regulatory submission (EMA/CHMP/QWP/BWP/259165/2019). Available at: https://www.ema.europa.eu/en/process-validation-manufacture-biotechnology-derived-active 502 substances-data-be-provided-regulatory-submission-scientific-guideline.
- Ph. Eur. General chapter on “Phage therapy medicinal products (5.31)
- ICH Q5D Derivation and characterisation of cell substrates used for production of biotechnological/biological products.
- Soleimani S. A Review of the Establishment of the Seed Lot System in the Production of Biological Products and Its Importance. Arch Razi Inst. 2022;77(6):2023-2035
- Hatfull GF, Hendrix RW. Bacteriophages and their genomes. Curr. Opin. Virol. 2011 1(4):298-303
- Chiang YN, Penadés JR, Chen J. Genetic transduction by phages and chromosomal islands: The new and noncanonical. PLoS Pathog. 2019;15(8):e1007878
- Ph. Eur. General chapter on “Bacteriophage potency determination” (2.7.38).
About The Author:
 Tim Sandle, Ph.D., is a pharmaceutical professional with wide experience in microbiology and quality assurance. He is the author of more than 30 books relating to pharmaceuticals, healthcare, and life sciences, as well as over 170 peer-reviewed papers and some 500 technical articles. Sandle has presented at over 200 events and he currently works at Bio Products Laboratory Ltd. (BPL), and he is a visiting professor at the University of Manchester and University College London, as well as a consultant to the pharmaceutical industry. Visit his microbiology website at https://www.pharmamicroresources.com.
Tim Sandle, Ph.D., is a pharmaceutical professional with wide experience in microbiology and quality assurance. He is the author of more than 30 books relating to pharmaceuticals, healthcare, and life sciences, as well as over 170 peer-reviewed papers and some 500 technical articles. Sandle has presented at over 200 events and he currently works at Bio Products Laboratory Ltd. (BPL), and he is a visiting professor at the University of Manchester and University College London, as well as a consultant to the pharmaceutical industry. Visit his microbiology website at https://www.pharmamicroresources.com.
