Packaging Good Manufacturing Practices (GMPs) For Medicinal Products
By Mark Durivage, Quality Systems Compliance LLC
Has your supplier of primary packaging materials for medicinal products established and implemented — and continued to maintain — an adequate quality management system (QMS)? Generally, most suppliers of packaging materials maintain an ISO 9001 QMS. However, the requirements of ISO 9001 may not provide the necessary levels of good manufacturing practices (GMPs) and rigor to ensure the packaging materials are adequate for their intended use.
to maintain — an adequate quality management system (QMS)? Generally, most suppliers of packaging materials maintain an ISO 9001 QMS. However, the requirements of ISO 9001 may not provide the necessary levels of good manufacturing practices (GMPs) and rigor to ensure the packaging materials are adequate for their intended use.
Types Of Packaging
There are three types of packaging: primary, secondary, and tertiary. Each type of packaging has unique requirements, risks, and intended use(s).
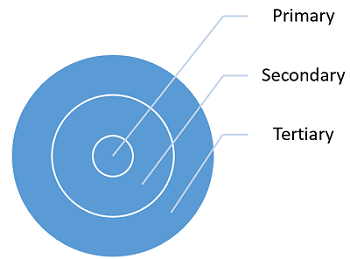
Figure 1: Types of packaging materials and their relationship
Primary packaging is designed to provide protection from excessive transmission of moisture or solvents into or out of the product, provide light protection for the product, provide additional microbiological protection by protecting the product from microbial intrusion, and provide protection from excessive transmission of reactive gases (atmospheric oxygen, inert headspace filler gas, or other organic vapors) into or out of the product. Examples of primary packaging include vials, syringes, ampules, stoppers, closures, bottles, pouches, and blisters.
Secondary packaging’s main purpose is for branding display, logistical purposes, and protecting and collating individual units during storage. Secondary packaging also includes packaging purposely made to display multiple product units for sale, which speeds restocking from storeroom to shelf; this packaging includes retail-ready packaging, shelf-ready packaging, or countertop display units. Examples of secondary packaging include pouches, boxes, and trays.
Tertiary packaging facilitates the protection, handling, and transportation of a series of sales units or secondary packages in order to group everything into unit loads during transit. This type of packaging is rarely seen by the consumer. Examples of tertiary packaging include boxes, totes, shrink wrap, and pallets.
Packaging components can be made using paper, paperboard, plastics (rigid and flexible), natural materials, metal, and glass and a multitude of modern production processes including injection molding, stretch blow molding, extrusion blow bolding, injection blow molding, compression molding, vacuum forming, extrusion, blown films, rolling, converting, etc.
ISO 15378:2017
ISO 15378:2017 Primary packaging materials for medicinal products — Particular requirements for the application of ISO 9001:2015, with reference to good manufacturing practice (GMP) specifies requirements for a QMS when an organization needs to demonstrate its ability to consistently provide products and services that meet customer and applicable statutory and regulatory requirements, with the goal of enhancing customer satisfaction through effective systems, continuous improvement, and commitment to customer and applicable statutory and regulatory requirements. ISO 15378:2017’s GMP principles in production and control of primary packaging materials are important for the safety of a patient receiving the medicinal product because of the direct contact between the packaging materials and the product.
ISO 15378:2017 follows ISO/IEC Directives Part 1 Annex L’s (formerly called Annex SL) structure. The annex stipulates how the International Organization for Standardization’s (ISO) Management System Standards (MSSs) should be written. Annex L’s purpose is to provide alignment and consistency of MSSs by providing a high-level structure for common core text, common terms, and core definitions.
According to ISO, the Annex L structure will:
- Put greater emphasis on leadership engagement
- Help address organizational risks and opportunities in a structured manner
- Address supply chain management more effectively
- Be more user-friendly for service and knowledge-based organizations
- Use a simplified language and a common structure and terms, which are particularly helpful to organizations using multiple management systems, such as those for environment, health, and safety or business continuity
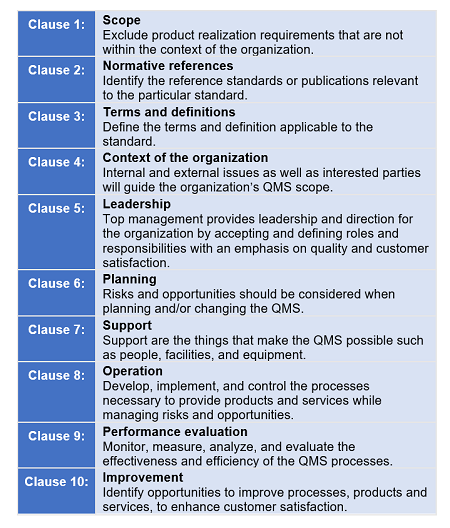
Figure 2: Annex L high-level structure
ISO 15378:2017 specifies GMP requirements applicable to primary packaging materials for a quality management system where an organization needs to demonstrate its ability to provide primary packaging materials for medicinal products that consistently meet customer and regulatory requirements. ISO 15378:2017 applies to the entire life cycle of primary packaging for medicinal products, including the design, production, quality control, labeling, handling, storage, and distribution.
Basic GMP Principles
The basic GMP principles are similar to those required in 21 CFR 210/211, 820, and 1270/1271. GMP guidelines are not prescriptive instructions on how to design, manufacture, label, store, and distribute products. GMP guidelines are general principles that must be applied. The QMS must ensure processes are controlled. Each company is responsible for determining the most effective and efficient methods to ensure compliance with GMP guidelines and general principles.
ISO 15378:2017 GMP requirements applicable to primary packaging materials include:
- The design, production, quality control, labeling, handling, storage, and distribution of primary packaging materials must minimize any risk to their quality.
- Manufacturing, testing, and storage facilities must be clean and hygienic.
- Facility design, operating principles, and environmental conditions must be controlled to prevent contamination and cross contamination of primary packaging materials.
- Manufacturing processes must be clearly defined, validated, and controlled to ensure consistency and compliance with specifications. Any changes to the process should be evaluated from a patient safety and product quality perspective, and any approved change that may affect the quality of the primary packaging materials should be qualified or validated as necessary.
- Standard operating procedures (SOPs) and work instructions (WIs) must be written in clear and unambiguous language using good documentation practices (GDPs).
- Operators should be trained to carry out production and quality control of primary packaging materials according to documented and approved SOPs and WIs.
- Records of manufacture and quality control activities are required to demonstrate that all the necessary steps required by the SOPs and WIs were properly executed and that the specified quality attributes of the primary packaging materials have been met. All deviations should be investigated and documented.
- Process should remain in a state of control throughout the product life cycle and improvements made as needed.
- Records of manufacture, labeling, testing, and distribution should be retained and be accessible in a format that can trace the complete history of a batch.
- A system must be available for recalling any batch from storage and/or distribution.
- Complaints should be evaluated, the causes of quality defects investigated, and appropriate measures taken with respect to the defective products and to prevent recurrence.
ISO 15378:2017 provides two very useful normative annexes:
- Annex C (normative) GMP requirements for printed primary packaging materials
- Annex D (informative) Guidance on verification, qualification, and validation requirements
for primary packaging materials
Packaging And Risk
Throughout ISO 15378:2017, the term “risk assessment” is referenced 22 times. ISO 15378:2017 requires risk assessments to determine the level of risk and the organization’s response to those risks. There are several commonly used tools available for performing a risk assessment, including failure mode effect analysis (FMEA), fault tree analysis (FTA), hazard analysis critical control point (HACCP), and hazard analysis and risk-based preventive controls (HARPC). FMEA and FTA focus on processing hazards, while HACCP and HARPC mainly focus on risks from intentional and unintentional physical, biological, chemical, and radiological contamination hazards.
Other factors that should be considered while assessing primary packaging material risks are acts of terrorism and other nefarious events such as illegal, falsified, intentionally contaminated, and/or counterfeit products.
Conclusion
Without GMPs, the quality of primary packaging materials may not be guaranteed, leading to potential quality issues, customer complaints, and regulatory actions. It is critical that you confirm your supplier of primary packaging materials for medicinal products has established, implemented, and maintains an adequate QMS and also considers the additional GMP requirements of ISO 15378:2017 to ensure the packaging materials are adequate for their intended use.
References:
- ISO/IEC Directives Part 1 15th Ed., International Organization for Standardization (ISO), May 2019.
- ISO 9001:2015 Quality management systems – Requirements.
- ISO 15378:2017 Primary packaging materials for medicinal products — Particular requirements for the application of ISO 9001:2015, with reference to good manufacturing practice (GMP).
About The Author:
 Mark Allen Durivage has worked as a practitioner, educator, consultant, and author. He is managing principal consultant at Quality Systems Compliance LLC, an ASQ Fellow, and an SRE Fellow. He earned a BAS in computer aided machining from Siena Heights University and an MS in quality management from Eastern Michigan University. He holds several certifications, including CRE, CQE, CQA, CSQP, CSSBB, RAC (Global), and CTBS. He has written several books available through ASQ Quality Press, published articles in Quality Progress, and is a frequent contributor to Life Science Connect. Durivage resides in Lambertville, Michigan. Please feel free to email him at mark.durivage@qscompliance.com or connect with him on LinkedIn.
Mark Allen Durivage has worked as a practitioner, educator, consultant, and author. He is managing principal consultant at Quality Systems Compliance LLC, an ASQ Fellow, and an SRE Fellow. He earned a BAS in computer aided machining from Siena Heights University and an MS in quality management from Eastern Michigan University. He holds several certifications, including CRE, CQE, CQA, CSQP, CSSBB, RAC (Global), and CTBS. He has written several books available through ASQ Quality Press, published articles in Quality Progress, and is a frequent contributor to Life Science Connect. Durivage resides in Lambertville, Michigan. Please feel free to email him at mark.durivage@qscompliance.com or connect with him on LinkedIn.
