Natural Killer Cells: Promise For Cancer Immunotherapy
By Booma Yandava, Principal Advisor at Biobridges
Historically, natural killer (NK) cells have received little enthusiasm. Unlike T and B cells, their origin is mysterious, and an understanding of their biology has been challenging to acquire. However, their role in immune surveillance against tumors and viral infection has never been in doubt. Moreover, it is now established that NK cells form a first line of defense against infections or host cells that have undergone stress, such as cancer cells.
Recent advances in Adoptive T-cell Therapies (ACTs) and human-induced pluripotent stem cells (hiPSCs), as well as in in vitro and in vivo models, have not only propelled the field of adoptive immunotherapies to the next level but also rekindled interest in harnessing the cytotoxic properties of natural killer (NK) cells due to challenges associated with T-cell based therapies that are discussed in the next section.
NK cells have come to re-exist in their own merit. Therefore, significant efforts have been undertaken not only to exploit the therapeutic properties of NK cells for innovative NK cell-based therapies but also to understand the immune circuitry around tumor growth and its control.
NK cells are considered highly suitable for clinical use because they can target not only cancer but also other areas, such as aging, due to their unique cellular properties. They may also serve as an alternative vehicle for Chimeric Antigen Receptor (CAR) engineering and manipulation. This article reviews NK-based therapies in the context of their associated challenges and proposes a future path for NK-based gene and cellular therapies.
Unique Anti-Tumor Properties of NK Cells
Although T-cell-based immune therapies have emerged as some of the most promising breakthroughs for cancer treatment, there is an increasing realization that these are not without limitations. In T cell-based therapies, both naïve and chimeric antigen receptor (CAR)-modified αβ T cells have shown promise for hematological malignancies. Predominantly one of two B-cell antigens, CD19 or BCMA, has been the target. Other antigens such as CD22 and CD19 /CD22 (CD19-22.BB.z-CAR), bispecific CARs are also being tried. However, with these T-cell CAR approaches, most widely seen adverse effects in clinical trials are neurological issues, cytokine release syndrome (CRS), loss of antigen and Graft Versus Host Disease (GVHD). Such effects highlight the challenges of engineering T cells with specificity and potency across multiple tumors, especially solid tumors. Thus far, CAR cell therapies have been tried only in advanced patient populations, although this is changing with recent trial enrollments. On the manufacturing side, T-cells have been challenging to manipulate for therapeutic use.
With these limitations with T-cells, attention has been turned towards harnessing NK cells for their innate, non-antigen specific immune response. It is well established that NK cells not only recognize tumor cells but also eliminate them through the cytotoxic mechanism of granzyme B and perforin.
Recent advances in NK cell biology and attempts to harness its non-antigenic, ‘ready to kill’ innate function have led to several strategies in NK cell therapies that aim to promote not only anti-tumor therapies but also in controlling inflammatory and autoimmune diseases and aging. However, the key question is whether T-cell manipulation approaches can be applied to NK cells, a question many teams are trying to answer.
Several articles have been published recently that review the biology of NK cells and their relevance to cancer. Rautela et al have reviewed the role of NK cells in cancer outcomes, along with the potential for NK cell-based immunotherapies to elicit anti-tumor activity in inflammation by invoking CD8+ T cell response. Weiner et al. in 2008 demonstrated that blocking NK inhibitory self-recognition promotes antibody dependent cytotoxicity in an anti-lymphoma therapy model (1). Barry et al have introduced NK cell–cancer cycle concept, thus enabling an understanding of the function of NK cells in relation to tumor abrogation (2).
Several groups have shown that NK cells express several Ig-like receptors and C-type lectin receptors that transduce both inhibitory and activating signals that enable NK cells to discriminate self from foreign or transformed cells. In addition to the expression of activating and inhibitory receptors, NK cells also express FcgRIIIa or CD16, receptors to the FC portion of antibodies, allowing them to eliminate cancer cells via mediating antibody-dependent cellular cytotoxicity (ADCC). This mechanism allows NK cells to recognize and eliminate antibody-coated cells. Exploiting and augmenting ADCC-mediated cell-killing has opened additional strategies for cancer treatment. Recently, monoclonal antibodies that have received FDA approval include Rituximab (anti-CD20), Daratumumab (anti-CD38), and Elotuzumab (anti-SLAMF7), and several more are under clinical development (1). Unlike T cells, NK cells offer the potential for ‘off-the-shelf’ cellular therapy, applicable not only for hematological malignancies but also for solid tumors.
Thus, besides tumor cell killing in a CAR-dependent manger, NK cells, possess a natural cytotoxic activity against tumor cells that can be activated in a CAR-independent mechanism, such as Natural Cytotoxic Receptors (NCR), NKG2D, CD226 and certain activating Killer cell immunoglobulin-like receptors (KIR – e.g., KIR2DS1, KIR2DS4 and KIR2DL4) receptors expressed on NK cells.
Thus, NK cells are uniquely suited for targeted cellular therapies for cancers.
Strategies to modulate NK cell functionalities for therapeutics and the clinical landscape
Remarkable progress has been achieved in adopting NK cells for cellular therapies, as evidenced by the number of clinical trials that are underway. A cursory analysis of clinical trials reveals there are at least 52 ongoing clinical trials with CAR-NK cells, most of them in the early phase or phase 1 or 2, targeting hematological malignancies (Fig. 1). This rapid increase in NK-based therapies in a short time has been made possible in part due to a keen recognition of limitations and opportunities of T-cell therapies.
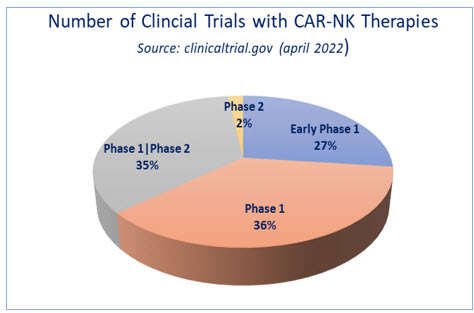
Fig. 1- On-going clinical trials with CAR-NK cell therapy. Source: clinicaltrials.gov
Currently, NK cell-targeting immunotherapies include adoptive transfer of NK cells, direct stimulation, recruitment of NK cells into the tumor microenvironment (TME), blockade of inhibitory receptors that are inhibitory to NK cell functions, and therapeutic modulation of the TME to enhance antitumor NK cell function and combinatorial therapies with PD-1 or other antibodies with NK cell therapies. Bispecific CD19/CD20 chimeric antigen receptor T cells show high efficacy in B cell malignancies (Fig.2). Rizwani et al and others have reviewed current strategies with CAR NK cells, highlighting that NK based therapies are only limited by one’s imagination (3).
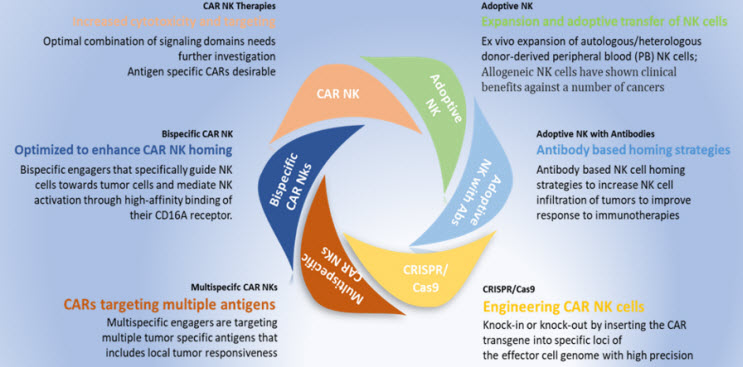
Fig. 2- Landscape of various approaches towards NK-cell based targeted immunotherapies
Adoptive transfer of allogenic NK cells has been demonstrated to be an efficient therapy for various cancers due to availability of source material from patients and low risk of graft versus host reactions. However, this approach is limited by the number of circulating NK cells in transferred patients that produced expected treatment outcomes. This is in part due to mismatches between inhibitory KIRs expressed on donor NK cells and recipients’ HLA ligands that trigger alloreactivity of transfused NK cells. Therefore, allogenic NK cells need to be depleted of T cells to prevent graft versus host reaction. Attempts to generate NK cells from umbilical cord blood (UCB), induced pluripotent stem cells (iPSCs), and developing NK cell lines such as NK92, derived from patients with lymphoma, have already proven as a viable source for NKC cells.
CAR-NK Therapy
CAR-NK cell therapy offers the advantage of augmenting the intrinsic tumor-killing properties of NK cells by using CAR-dependent mechanisms for obfuscating the immune escape mechanism by tumors. Katy Rezvani’s group has published the clinical results of 11 patients receiving CAR-NK cells derived from umbilical cord blood (UCB) for targeting CD19-expressing B-cell malignancies (NCT03056339). 73% of these patients showed a clinical response, seven of whom experienced complete remission. This trial and other trials by several groups indicate that CAR-NK cells might offer a promising therapeutic avenue. The first clinical trial targeting CD19+ R/R B cell malignancies using CAR-NK cells (NCT05020678) has been approved, and the results are expected in early 2023.
At the 2022 AACR, two groups presented data based on off-the shelf allogeneic CAR-NK cell therapy derived from umbilical cord blood. Cytoimmune Therapeutics, in collaboration with City of Hope, demonstrated enhanced cytotoxicity and IFN-gamma secretion against FLT3+ AML in vitro and enhanced survival of mice engrafted with FLT3+ AML of FLT3 CAR_s15 NK cells, which will be further investigated in clinic, offering better hope for AML patients (4).
Results presented at the same AACR meeting from single-center phase I-II trial (NCT04074746) evaluating AFM13 complexed with cord blood-derived NK cells in patients with relapse/refractory CD30+ lymphoma indicated tolerability and high activity against lymphoma with no cytokine release syndrome or the graft vs. host reaction typically observed with CAR-T therapies. This approach is being investigated in additional trials (5).
NK Cell Engagers
Of special interest are NK cell engagers (NKCEs) that are designed with fragments of tumor specific antibodies and activating receptors on NK cells such as CD16 with a goal of bringing tumor cells and NK cells together and trigger cytotoxicity against tumors. There are also approaches that have demonstrated efficacy and less toxicity in preclinical setting with tri-specific engagers such as NKp46 and CD16 on NK cells and tumor specific antigens. Thus, tri- and multi-specific target engagement approaches have been demonstrated to be promising approaches. Figure below summarizes current approaches utilizing various targets
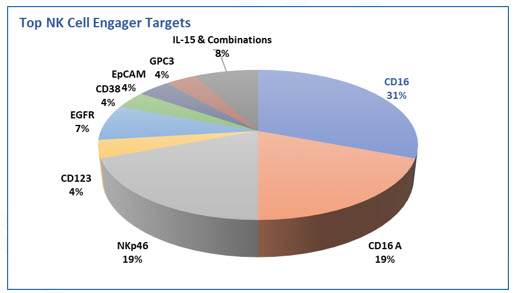
Fig. 3 - NK cell engagers showing CD16 as the predominate target
Challenges and Opportunities
Cell therapy with NK cells is considered to be a safer alternative to T cell-based therapies for cancer treatment. Numerous articles have reviewed NK cells and their role in immunosurveillance. Two fundamental approaches are being tried: NK cells derived from a autologous source and NK cells derived from a heterologous source. Efforts are being channeled in engineering and developing NK cells that will not only enhance their cytotoxic function but also pave the way for clinical solutions.
However, along with the opportunities, challenges remain. NK biology is complex, as NK cells suppress tumor cells through a variety of effector mechanisms, including the perforin/granzyme-containing granule-mediated pathway, death-receptor pathway and IFN-γ-mediated pathway. NK cell function is regulated by a fine balance of activating signals and inhibitory signals through multitude of receptors that are expressed on the cell surface, and each receptor has its own role in controlling tumors. These roles are still being elucidated. NK cells are also heterogeneous in the population and acquire different phenotypes depending on signaling cues and developmental stages, further complicating NK biology. In addition, NK cells may also influence the development of innate immunity through modulations of antigen-presenting dendritic cells. The major challenge with harnessing NK cells for cancer treatment is that many tumors are sparsely infiltered with NK cells (Barry et al 2020).
Nevertheless, it has been shown that tumor cell recognition through NK cell receptors is superior, with no requirement for antigen stimulation, an attractive feature for developing off-the-shelf utility. Emerging clinical trials demonstrate that CAR-NK cells have low toxicity with high response. Current NK cell-based therapies have shown promise for hematological malignancies in clinical settings and have demonstrated efficacy in pre-clinical settings with various modifications towards off-the-shelf NK-based therapies. The field recognizes the importance of off-the-shelf NK cell therapy not only for cost reduction but also for improving safety measures. It is anticipated that many more clinical results will be shared in the future. It is important to understand and utilize NK cells for their cytotoxic function for cancer treatment. Moreover, it is crucial to gain an understanding of NK cell differentiation pathways and immune modulatory functions that would lead to better and more cost-effective NK-based therapies.
Acknowledgements:
Many Thanks to Dr. Lee Sayers, Director - Solid Organ Oncology, Regeneron Pharmaceuticals for his insights and critical review.
About the Author :
Booma Yandava, M.S, MBA, CGBP, CA-AM brings her expertise in Biologics, Cell and Gene Therapy based drug development in leading and providing guidance on long-term strategic growth opportunities with business development and partnering expertise. Currently she is at Samyang Biopharm USA, Director, Business Development.
References:
- https://doi.org/10.4049/jimmunol.180.9.6392
- https://doi.org/10.1038/s41590-020-0728-z
- https://doi.org/10.1002/cti2.1274
- AACR, 2022 LB102 / 9
- AACR. 2022, CT00
