Multi-Column Chromatography

Downstream bioprocessing steps have long held challenges for drug manufacturers, particularly the chromatography step for purification of monoclonal antibodies (mAbs).
The complexity of traditional chromatography options as well as the high cost of the Protein A resin required have driven the search for a more flexible, cost-effective and convenient alternative.
PROTEIN A RESIN COSTS ARE A DRIVING FACTOR FOR MCC
Protein A capture chromatography is a crucial step in all downstream processing of mAbs. This purification step provides a highly effective and robust means of removing host cell proteins and other impurities from the harvested cell culture fluid (HCCF). Additionally, Protein A is a platform process step for mAbs that allows for more rapid development and scale-up of purification processes.
However, Protein A resin is expensive, which means the costs associated with its use in chromatography can be very high. An upfront purchase of a large quantity of resin is typically required to produce clinical supply material at each phase of the development lifecycle. Thus the cost burden to get a new drug to the market, associated solely with resin procurement, can be substantial.
MULTI-COLUMN CHROMATOGRAPHY VS. BATCH CHROMATOGRAPHY
Multi-column chromatography (MCC) splits the load zone into two columns in series, allowing for product breakthrough from the first column to be captured by a second column. This reconfiguration of the load zone results in the possibility of a greater binding capacity for the first column compared with batch chromatography. MCC also facilitates simultaneous processing fully loaded columns through the wash, elution and regeneration phases, thereby allowing uninterrupted processing of feed material since a newly regenerated column moves into the load phase as soon as the target load volume on a column is reached. Together, these aspects of MCC allow for a reduction in the quantities of Protein A resin required, often by up to ~80%.
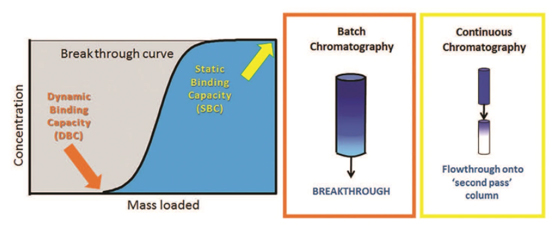
This reduction in resin requirement is significant because of the cost of Protein A resin. In some cases, the cost of Protein A resin is high enough to create a financial barrier, preventing small and emerging pharmaceutical companies from having enough capital to generate clinical supply material.
IS MCC TECHNOLOGY RIGHT FOR YOUR PROJECT?
MCC technology still use the same sequence of steps used in batch chromatography, but simply accomplishes this through parallel processing of several small columns rather than one large column. Hence, MCC technology is also an option for manufacturers wishing to switch from batch to continuous processing at any phase of clinical drug development.
The reduction in resin associated with implementing MCC technology as part of the downstream process can significantly affect both a project’s up-front and final development costs, especially when large quantities of resin are required for just a few clinical supply runs.
Recognizing the financial burden associated with generating clinical materials, AGC Biologics offers multi-column chromatography technology, to help their clients reduce clinical manufacturing cost through improved resin utilization.
