Mitigating Risks To Global Distribution Of COVID-19 Vaccines And Therapeutics
By Narendra Chirmule (SymphonyTech Biologics), Whitney Winters (West Pharmaceutical Services), Stephan Roenninger (Amgen), Elisabeth Vachette (Sartorius), and Maura Kibbey (U.S. Pharmacopeia)
As of July 31, 2020, the FDA’s Coronavirus Treatment Acceleration Program had reported 570 SARS-CoV-2 drug development programs in planning stages and 270 FDA-reviewed trials.1 Meanwhile, the World Health Organization’s Aug. 20, 2020 draft landscape listed 30 candidate vaccines in clinical evaluation and 139 in preclinical evaluation.2 While these numbers illustrate the extraordinary efforts of the global community, they only provide a snapshot of the continuing developments aimed at addressing the current coronavirus pandemic.
A growing number of novel and repurposed therapeutics are in development or clinical trials to combat multiple aspects of associated clinical pathologies (e.g., antivirals, immunomodulators, antibodies that may provide passive short-term immunity, etc.) as scientists and clinicians gain a better understanding of the virus. On the vaccine side, the research-based pharmaceutical industry is trying a multipronged approach to stimulate the immune system by using both traditional approaches (i.e., protein subunits, inactivated viruses, and virus-like particles) and novel platforms that are based on delivery of mRNA, DNA, or non-replicating viral vectors that can trigger the body to make the viral proteins themselves. The typical timelines to design, robustly manufacture, and consistently produce high-quality vaccines from these diverse technologies can vary from months to many years, not including the time it takes to demonstrate safety and efficacy. Prior to this pandemic, a vaccine development program for a novel target from inception to licensure has averaged 12 years.
As researchers and manufacturers race to develop the next therapy or vaccine in the fight against COVID-19, demonstrating this quality, safety, and efficacy is critical. However, all the work and investment in developing a COVID-19 therapy and vaccine can be lost in the last mile. Managing the manufacturing of large quantities with many steps, the supply chain, and distribution challenges to deliver these therapies and vaccines, as well as other existing therapies, to patients is just as important. This need for a robust supply chain, from the API starting material with all components, until the final packaged and registered drug product, is particularly true under the accelerated timelines that the world demands to solve this pandemic. Mitigating manufacturing, supply chain, and distribution risks is not new. Other causes, such as natural disasters (e.g., earthquakes, volcanos, hurricanes, fires) or business and economic effects, can also lead to interrupted supply at any time.
Many vaccines and therapies, particularly biologics, require administration by injection and, therefore, assurance of sterility and container integrity is critical. Consequently, proper manufacturing and storage and transportation conditions, especially temperature, must be monitored and controlled to maintain safety, quality, and efficacy — from manufacture to patient delivery. Many of the COVID-19 candidate therapies may have the same challenges as these other injection products and compete for the same manufacturing capacities and packaging materials, e.g., pharmaceutical-grade glass vials, syringes, bags, filters, stoppers, etc.
All partners in the vaccine supply chain and organizations (e.g., USP, EDQM, WHO, Gavi, CEPI, PhRMA, EFPIA, etc.) recognize that these challenges must be assessed and mitigated. Increased monitoring and corrective and preventive actions of supply chain issues and technical support for analytical challenges need to be addressed.3 To further support this mission, the U.S. Pharmacopeia (USP) organized a webinar with a panel discussion, Bringing COVID-19 Vaccines and Therapeutics to the Global Community, and then the panel members collaborated to prepare this report.4 Based on our expertise in biologics development, quality, distribution, raw material supply, and manufacturing, we shared best practices and ways to control risks to bringing products to patients around the world. This article is a summary of those discussions.
Reliable Manufacturer And Supplier Networks Are Critical
Manufacturers establish processes to control supply and distribution risks, ensuring the availability of drugs and vaccines. As illustrated in Figure 1, manufacturers can control risks to supply by:
- Developing a reliable manufacturing and supplier network, consisting of internal manufacturing capabilities and contract manufacturing organizations, to buffer against disruptions;
- Investing in preventive measures— e.g., infrastructure, updated technology, business continuity plans, inventory management, diversification;
- Establishing an agile warehouse and allowing a direct to patient supply chain strategy with multiple distribution sites and warehouses; and
- Preemptively addressing manufacturing, business, economic, and security risks to distribution.
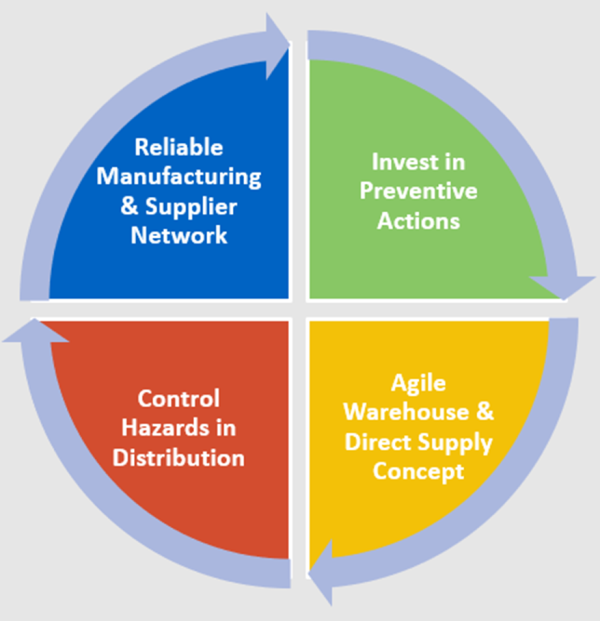
Figure 1: Controlling risks to ensure worldwide product availability
The level of effort and formality will vary based on the level of risk and will differ by location, region, and culture. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has produced and is maintaining numerous guidelines that can be consulted related to the supply chain, such as its quality guidelines, Q9 Quality Risk Management, Q10 Pharmaceutical Quality System, and Q12 Lifecycle Management.5 WHO also provides important information on the supply chain and logistics.6 Identifying hazards and assessing risks early that could potentially lead to failures is important.7 Constant, transparent communication between all parties in the supply chain, especially suppliers and sponsors, is critical to meet tight timelines and meet expectations from all parties, including patients.
Demonstration Of Vaccine Safety And Efficacy Must Be Rigorous
It was emphasized that vaccine efficacy hinges on producing a robust immune response. Key antigen targets include the viral spike, envelope, and membrane proteins. Some vaccines deliver the proteins themselves, while others trigger the body to manufacture these antigens. The robustness of an individual’s immune response to these vaccines will be multifactorial: genetics, innate immune responses, preexisting T-cell responses, and overall health will all contribute to an individual’s ability to produce neutralizing antibodies and cytotoxic T-cell responses after vaccine administration. The varied approaches to vaccines in development may trigger different immune pathways impacting short-term efficacy as well as long-term protection. The various vaccine platforms will need to be tested in different populations, and some vaccines may work better in one population versus another.
Centralized And Decentralized Manufacturing Are Both Options
There are pros and cons to both centralized and decentralized manufacturing. Decentralized manufacturing can be attractive to ensure local production of needed medicines in high quantity, but if high-quality raw materials are difficult to obtain or if manufacturing experience and knowledgeable staff are lacking, then it can be challenging to produce high-quality vaccines using a decentralized model. While centralized manufacturing may obviate some of these challenges, this model can lead to challenges with supply of the finished vaccines to all regions where they are needed if distribution chains are disrupted. However, this can be managed with appropriate supply strategies, including inventory and decentralized warehousing and inventory management depending on the shelf life. Suppliers and manufacturers must be clear on what the quality requirements are for scaling up production of vaccine components, vaccine products, and the container systems used to store those products. Without clear communication between license holders, regulators, suppliers, manufacturers, and distributors, local supply chains can collapse and delay distribution of vaccines to patients. Manufacturers, distributers, and pharmacies can also buffer against some of these risks by keeping safety stocks.
There are single-use and continuous manufacturing technologies that can support more rapid manufacturing scale-up, sterility, and potentially a decentralized manufacturing approach that many companies are adopting in collaboration with experienced suppliers. At least initially, the USP panel believes that a centralized manufacturing approach in collaboration with experienced distribution partners and the regulatory authorities can effectively deliver vaccines around the globe, even with needed but challenging cold chain requirements. Whether centralized or decentralized manufacturing strategies are chosen, the extremely short timelines expected for COVID-19 vaccine production and safe distribution can lead some manufacturers to choose technologies like single-use or continuous manufacturing solutions. Both techniques can usually be implemented and validated much faster than traditional stainless-steel facilities, in some cases in less than 12 months. Since they are single-use, they also don’t have the cleaning and re-sterilization needs, thereby reducing the risk of microbial contamination and cross contamination, as well as being environment-friendly due to less use of power, water, emissions, etc. As manufacturers scale up, these options can grow in parallel while reducing risk if a product doesn’t make it to market. If a product is approved, rapid commercial production can start in a pilot facility and then a dedicated stainless-steel facility can be extended or rapidly built if needed, depending on the demand.
Creative Supply Chain Strategies May Be Necessary
As described above, strong partnerships and open communication between manufacturers and their suppliers and customers, as well as with regulators and inspectors, are critical but can be challenging when travel is prohibited. Establishing agreed upon protocols to securely audit a new supplier has become more common. These include virtual audits and inspections, as well as reliance on inspection by recognized authorities (e.g., member inspectorates of the Pharmaceutical Inspection Co-operations-Scheme [PIC/S] implementing mutual recognition agreements). When setting these up for the first time, the panel recommended that partners consult the guidance, including GMPs created through the PIC/S,8 and this guidance be followed to support their discussions.
Storage and cold chain considerations will vary depending on which vaccines are approved and will impact patient delivery options. If a vaccine or other drug product requires storage at minus 80 degrees C or even colder, it is unlikely that every physician can manage storage in an ultralow freezer and administer vaccines in their clinic. If a vaccine must be stored refrigerated, its potency may be greatly decreased if subjected to freeze-thaw cycles by incorrect storage on ice packs. Cold chain channels have been effectively set up over the years for many other sensitive therapeutics and utilizing existing channels will decrease risk for these vaccines.
When so many new vaccines and therapeutics for COVID-19 are simultaneously developed, this can impact existing supply chains for previously approved therapeutics as well. Increasing capacity for labor and equipment to scale up production and distribution of a new vaccine must be balanced with fulfilling current needs for existing medicines, especially biologics, which can use the same raw materials and need the same quality controls as the vaccines in development. Mass production and distribution will need multiple partnerships and open communication between industry, testing labs, regulators, and others to maintain adequate supply and quality.
Also, as it is today, for every new pharmaceutical product developed, counterfeit products may enter the distribution channels, especially if it is a high-demand product that isn’t accessible to everyone, such as a newly approved vaccine. If a poor-quality or counterfeit vaccine is administered to a patient, it could cause serious harm, either due to the material itself being harmful or to its lack of efficacy, leading to a lack of protection against COVID-19. This could further erode the public’s trust in vaccines even more than that seen today. Regulations have been implemented in numerous regions to provide oversight and prevent falsified products entering the market.9
Conclusion
Worldwide collaborative efforts are needed to rapidly solve this pandemic without putting patients at risk by the goal of reducing the typical biologics development program timeline from around 12 years to slightly over 12 months.10 By developing real-time solutions for managing supply chain disruptions through effective communication, the healthcare community can help ensure that people around the world receive high-quality vaccines and therapeutics that are accessible, safe, and effective.
Notes:
- https://www.fda.gov/drugs/coronavirus-covid-19-drugs/coronavirus-treatment-acceleration-program-ctap
- https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- https://www.statnews.com/2020/06/02/covid-19-exposed-cracks-global-medicines-supply-chain/
- https://www.usp.org/course/bio-vac-01
- https://www.ich.org/page/quality-guidelines
- https://www.who.int/immunization/programmes_systems/supply_chain/en/
- E. Ramnarine, M. Jornitz, M. A. Long, K. O'Donnell, S. Rönninger, C. Smalley, A. Vinther, Risk based approach for prevention and management of drug shortages, PDA Technical Report, 68, 2014, 1-45. https://www.pda.org/scientific-and-regulatory-affairs/regulatory-resources/drug-shortage, accessed Sept 23 2020
- https://picscheme.org
- For example, EU directive, Prevention of the entry into the legal supply chain of falsified medicinal products, 2011/62/EU, 11. June 2011. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:174:0074:0087:EN:PDF
- https://www.ohe.org/publications/rd-cost-new-medicine#
About The Authors:
 Narendra Chirmule, Ph.D., is the co-founder and CEO of SymphonyTech Biologics, a data analytics company focused on engineering solutions to biology. As former head of R&D at Biocon, and having held leadership positions in Amgen and Merck Vaccines, he has contributed to clinical development of vaccines and biopharmaceuticals. He did a Ph.D. on development of a leprosy vaccine at the Cancer Research Institute, Mumbai; post-doctoral studies on pathogenesis of AIDS at Cornell University Medical College-North Shore Hospital; and teaching and research as assistant professor in gene therapy at the University of Pennsylvania. Chirmule is also on the NIH advisory committee for HIV vaccines.
Narendra Chirmule, Ph.D., is the co-founder and CEO of SymphonyTech Biologics, a data analytics company focused on engineering solutions to biology. As former head of R&D at Biocon, and having held leadership positions in Amgen and Merck Vaccines, he has contributed to clinical development of vaccines and biopharmaceuticals. He did a Ph.D. on development of a leprosy vaccine at the Cancer Research Institute, Mumbai; post-doctoral studies on pathogenesis of AIDS at Cornell University Medical College-North Shore Hospital; and teaching and research as assistant professor in gene therapy at the University of Pennsylvania. Chirmule is also on the NIH advisory committee for HIV vaccines.
 Stephan Roenninger, Dr.-Ing., works in the Quality External Affairs organization in Amgen. He collaborates cross-functionally with internal and external stakeholders on topics regarding quality management, good manufacturing and distribution practice, and CMC by shaping regulatory guidelines, disseminating intelligence, and providing education on quality and regulatory matters. Roenninger also represents Amgen in EFPIA and represents EFPIA on the ICH training committee and the ICH-QDG. In PDA he is an elected director of the board and chair of the Regulatory Affairs and Quality Advisory Board and the Inspections Trends Interest Group. Roenninger holds a Ph.D. and engineering degree in organic chemistry from the Technical University of Darmstadt, Germany.
Stephan Roenninger, Dr.-Ing., works in the Quality External Affairs organization in Amgen. He collaborates cross-functionally with internal and external stakeholders on topics regarding quality management, good manufacturing and distribution practice, and CMC by shaping regulatory guidelines, disseminating intelligence, and providing education on quality and regulatory matters. Roenninger also represents Amgen in EFPIA and represents EFPIA on the ICH training committee and the ICH-QDG. In PDA he is an elected director of the board and chair of the Regulatory Affairs and Quality Advisory Board and the Inspections Trends Interest Group. Roenninger holds a Ph.D. and engineering degree in organic chemistry from the Technical University of Darmstadt, Germany.
 Elisabeth Vachette is head of product management for bags/mixing/tanks within the Single Use Fluid Management Technologies department at Sartorius based in Aubagne, France. Since joining Sartorius Stedim Biotech in 2000, she has been responsible for quality, production, engineering, and now product management, providing a broad view of the functionalities of single-use solutions development, marketing, qualification, and manufacturing. She is an active member of scientific committees for PDA conferences and is member of the BPSA Board of Directors.
Elisabeth Vachette is head of product management for bags/mixing/tanks within the Single Use Fluid Management Technologies department at Sartorius based in Aubagne, France. Since joining Sartorius Stedim Biotech in 2000, she has been responsible for quality, production, engineering, and now product management, providing a broad view of the functionalities of single-use solutions development, marketing, qualification, and manufacturing. She is an active member of scientific committees for PDA conferences and is member of the BPSA Board of Directors.
 Whitney Winters is the senior director of strategic marketing, container systems, for West Pharmaceutical Services, Inc. She joined West in 2007 as a pricing specialist and has since worked as an account manager, sales manager, and senior sales director for emerging biologics, where she led a team that assisted clinical-phase biotech companies with containment and delivery solutions. In her current role, she creates the strategy to drive promotion and adoption of high-value programs, pharmaceutical device systems, and custom product development. Winters received her MBA from Pennsylvania State University, and a B.A. with a specialization in chemistry from Northwestern University.
Whitney Winters is the senior director of strategic marketing, container systems, for West Pharmaceutical Services, Inc. She joined West in 2007 as a pricing specialist and has since worked as an account manager, sales manager, and senior sales director for emerging biologics, where she led a team that assisted clinical-phase biotech companies with containment and delivery solutions. In her current role, she creates the strategy to drive promotion and adoption of high-value programs, pharmaceutical device systems, and custom product development. Winters received her MBA from Pennsylvania State University, and a B.A. with a specialization in chemistry from Northwestern University.
 Maura Kibbey, Ph.D., is senior scientific fellow for education and training in USP’s Global Biologics department. She leads development of courses, workshops, and forums to engage USP’s biologics stakeholders. This role builds on her previous responsibilities directing USP scientists developing compendial standards. Before joining USP, Kibbey worked for several biotechnology and diagnostic companies in the Washington, D.C., area in scientific, management, marketing, and business development roles, as well as performing cancer research at the National Institutes of Health. She has published over 40 peer-reviewed articles and has been an invited speaker or workshop organizer for numerous scientific conferences.
Maura Kibbey, Ph.D., is senior scientific fellow for education and training in USP’s Global Biologics department. She leads development of courses, workshops, and forums to engage USP’s biologics stakeholders. This role builds on her previous responsibilities directing USP scientists developing compendial standards. Before joining USP, Kibbey worked for several biotechnology and diagnostic companies in the Washington, D.C., area in scientific, management, marketing, and business development roles, as well as performing cancer research at the National Institutes of Health. She has published over 40 peer-reviewed articles and has been an invited speaker or workshop organizer for numerous scientific conferences.
The US Pharmacopeia (USP) is an independent, nonprofit, scientific organization that collaborates with the world's top experts in health and science to develop quality standards for medicines, dietary supplements, and food ingredients. Through our standards, advocacy and education, USP helps increase the availability of quality medicines, supplements and food for billions of people worldwide.
