Illuminating the Need for Standards in Regenerative Medicine and Advanced Therapy
By Robert Shaw, Executive Director Standards Coordinating Body for Regenerative Medicine

With significant approvals and a robust pipeline of potential treatments for serious diseases, the future of cell and gene therapy is bright. According to data from the Alliance for Regenerative Medicine, more than 1220 clinical trials underway; 152 are in Phase III (ARM SOTI, Jan 2021), and almost $20 billion dollars was raised in 2020 (ARM SOTI, Jan 2021). Despite the progress to date, major questions and obstacles remain for the industry. How do we address commercialization/industrialization (scale-up or out), how do we address costs, and how do we accelerate innovation to help support these goals? While it may not seem obvious, one answer is standards and standardization.
The Standards Coordinating Body
The Standards Coordinating Body (SCB), based in Gaithersburg, Maryland, was formed in 2017 to focus on the areas of standards, standardization, and the standards development process for the regenerative medicine and advance therapy sectors. The organization began as an initiative of ARM and other regenerative medicine stakeholders and industry. In 2016, the National Institute of Standards and Technology (NIST) and SCB established a Memorandum of Understanding (MOU), forming a partnership to jointly advance standards for the regenerative medicine community. This MOU provides a mechanism for more cooperation with other U.S. agencies to work with industry, standards development organizations, and other stakeholders.
SCB is now a fully independent non-profit organization. Recognizing the importance of standards in this sector, The U.S. Food and Drug Administration (FDA) supports SCB with funding to increase and accelerate the number of regenerative medicine standards being advanced in the field. These activities are intended to continue supporting the vision of the 21st Century Cures Act of 2016
SCB’s mission is to educate the regenerative medicine and advanced therapies ecosystem on the importance of standards and bring stakeholders together to prioritize and catalyze standards development, harmonization and adoption across the industry (Figure 1).
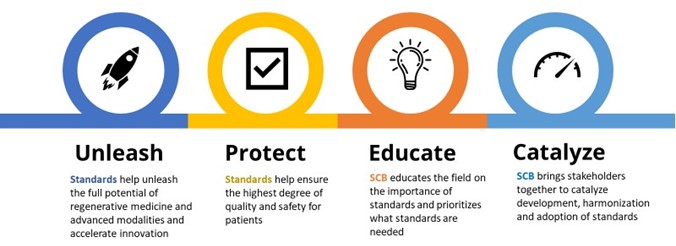
Figure 1. SCB is focused on educating the sector on the importance of standards and accelerating their development.
The Case for Standards
Regenerative medicine and advanced therapies present complex challenges related to the consistency of scientific protocols, testing, and product quality and performance. Standards development has the potential to overcome a broad array of challenges and benefit stakeholders—including industry, academia, clinicians, and regulators. (Figure 2).

Figure 2. Standards will have an impact across all aspects of cell and gene therapies.
In the past, standards development in the life sciences has required years. This can be attributed to the passive process which has been primarily based on volunteer contributors from industry. SCB accelerates the process by focusing on high priority/high impact standards and catalyzing the process. Without a coordinating body like SCB, these pre-development steps can take up to six years; with SCB’s support, these steps take as little as six months to one year (Figure 3).
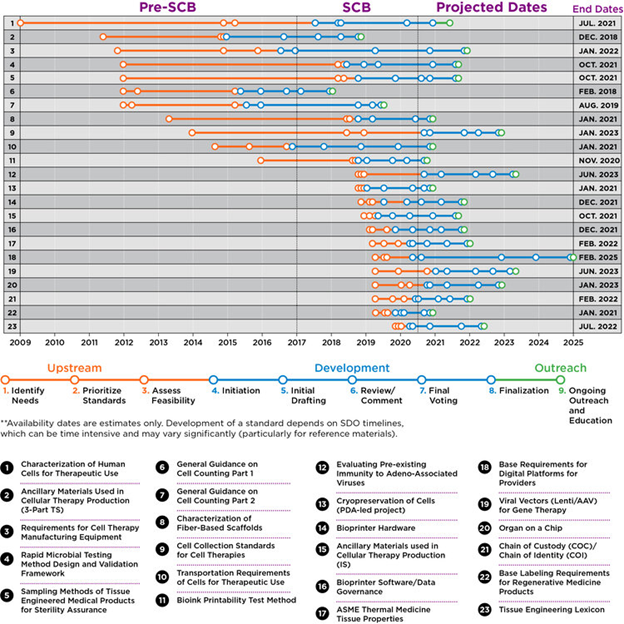
Figure 3. Overview of standards currently in development and acceleration of the development process by SCB.
A recent article by Armon Sharei, CEO of SQZ Biotechnologies on the potential and future of gene therapy noted that “Cell therapies today are in their infancy relative to what they could become due in part to two major constraints: the difficulty in engineering cell functions; and the barrier presented by manufacturing cell therapies at scale.”
Standards, applied thoughtfully and phase appropriately, can provide the basis for how to approach and progress in these areas. SCB has heard from many of its stakeholders that simply finding standards was a challenge. In our Landscape Report for Regenerative Medicine, we reported that there were more than 200 standards in place across cell therapy, gene therapy and tissue engineering, established by a diverse roster of standards development organizations. Unfortunately, there was no connective tissue bringing all of the standards together nor an organization with visibility to what was in development or a process to engage the necessary stakeholders.
The Value of Standards
There are three primary reasons to implement standards in your processes: cost, time/speed and efficiency. Standards developed for cell counting and viability offer an example of their impact. Both are critical parameters for determining dose and efficacy, are ubiquitous in terms of their need and technologies exist for both. Yet these areas continue to be highlighted by regulatory authorities and industry as needed areas for standardization.
An ISO standard exists for implementation of cell counting for both allogenic and autologous products (ISO 20391-1:2018); relatively few companies, however, are utilizing these in their processes. We can look at this from two perspectives, within a process, and at regulatory review.
As many processes are either transferred from academia to innovator, and/or innovator to contract manufacturer, cell counting becomes especially important within a specific process. How does one ensure that the number of cells determined (e.g. between two sites) is the same? What equipment/technology is used for enumeration? Are these the same? What reference material is used to facilitate tech transfer? A reference material provides both a cost and time benefit to innovators in that they will not have to generate the material or methods required to produce it. In addition, use of a reference material eliminates the redundancy of each company having to develop their own.
As many companies are developing similar products for therapeutic use (e.g. mesenchymal stem cells, CAR-T cells) dosing is still a primary measure to be determined in clinical trials and subject to regulatory review. The consistency of cell counting will be one question that must be addressed especially in autologous therapies as patient and process variability will likely have impact on the cell product. What reference material has or should be used, and what process of assay validation has been put in place to help address product quantification? If a standard material and/or standard method has been used and documented in the CMC, this provides the regulatory authorities the reference by which to judge the quality of data supporting this aspect. Without a standard material or standard method, the regulatory authorities will look for each innovator to provide date that will need to be empirically produced. This also makes it much more challenging for the regulatory authorities to compare data across other similar products.
Viability assessment is similar. Whether part of a tech transfer process, comparability assessment (for process improvements/scale-up) or for regulatory review, a common approach to viability will aid cost, time and efficiency for an organization. Important questions in the definition of viability include whether cells need to be alive, respiring, and/or functionally active (producing the markers or components required for efficacy) have yet to be definitively addressed, and “simple” viability is still an area of needed standardization.
SCB Resources and How to Get Engaged
Through active engagement of the regenerative medicine and advanced therapies communities, SCB is addressing the most critical areas of need with broad input and wide consensus so we can collectively unleash the full potential of modalities.
In order to further help the industry with access to standards, SCB developed the Regenerative Medicine Portal that provides a searchable database to find and link directly to these standards.
SCB has gathered feedback and input from more than 250 regenerative medicine stakeholders to develop our Needed Standards Report. A diverse set of stakeholders representing industry, healthcare, government and academia identified 44 areas where standards were needed (Figure 4). The areas were assessed by a group of industry and regenerative medicine community participants and ranked based on impact versus urgency.
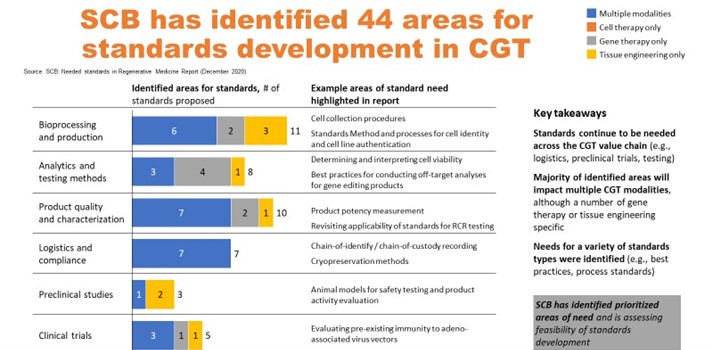
Figure 4. Identification of 44 areas for standards development.
The top areas of need are currently being assessed by stakeholders for feasibility and determinations will be made as to whether they should proceed into the standards development process. Figure 5 provides an overview of the feasibility assessment process.
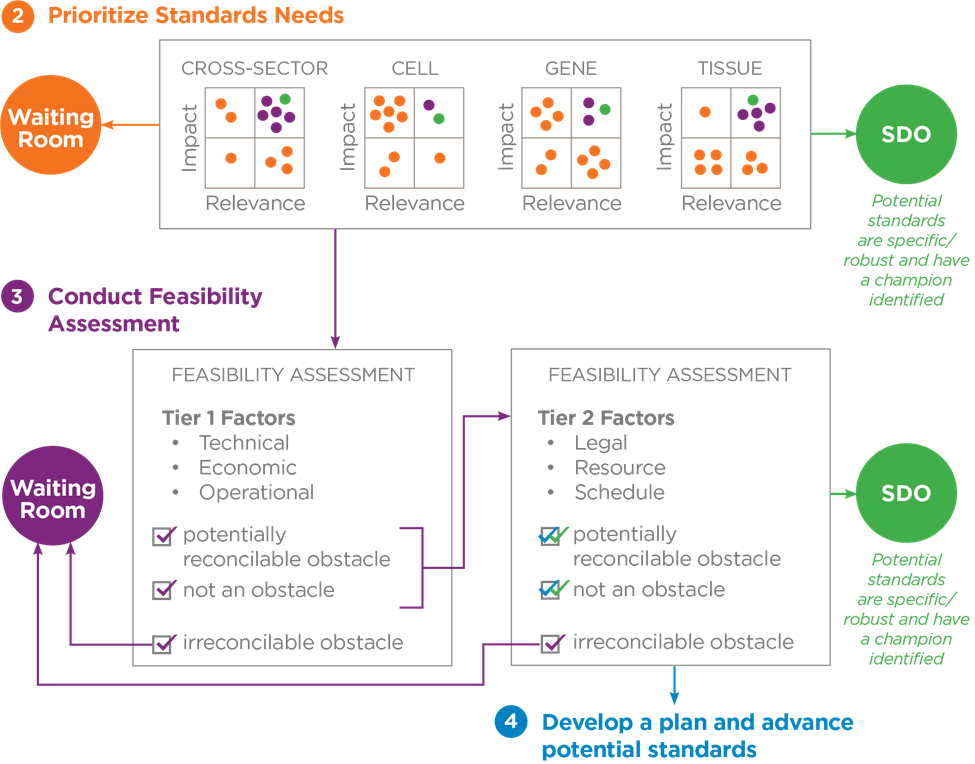
Figure 5. Overview of the feasibility assessment process
SCB’s projects and initiatives are organized and advanced within a set of working groups focused on gene therapy, cell therapy, tissue engineering and cell-based drug discovery. Each working group consists of expert representatives from industry, academia, clinical practice, and other relevant stakeholder groups, who identify and assess needs for standards or best practices that can support the advancement of the regenerative medicine field.
Once the sector working group has identified the need for a standard, SCB may then form a project working group to further assess the potential standard’s priority and feasibility and ultimately coordinate its advancement through a relevant standards development organization (SDO).
In addition to the recently launched portal and establishing working groups, SCB is developing an advisory service to help innovators both map standards to their processes and help identify phase appropriate implementation plans. One of the most important areas though is who is responsible for assessing the need, priority areas and timing of standards implementation in product development. The earlier this resource can be identified, the easier it will be for companies to develop a strategy for implementation, which will result in savings of cost and time overall.
The Way Forward
The regenerative medicine and advanced therapy industries have the potential to deliver tremendous impact on patients. Significant investment has flowed into the sector and there is clear support for its progress. Standards and standardization can help industry address key issues such as cost, time and efficiency through implementation of a standards strategy (mapping of their processes) and plan. SCB is an organization focused on continuing to accelerate the standards development process, inform and educate the field by making information as readily available as possible, and is looking to help industry implement standards in the most efficient and appropriate ways.
