Getting Out Of The Doldrums: Analyzing & Kickstarting Organizational Quality Maturity
By Samantha Atkinson, executive vice president and principal consultant, NSF

It is well-known that regulatory authorities across the world are delving more deeply into the ongoing supply disruptions caused by quality system issues. These challenges must be resolved for businesses to thrive in compliance with the ever-changing regulatory landscape.
A number of regulatory authorities have highlighted culture and behaviors as clear factors in quality system issues. Indeed, the U.S. FDA has been considering1 the best mechanism to operationalize its quality management maturity (QMM) assessment as part of its regulatory oversight of the sector. The FDA describes2 QMM as the state attained when drug manufacturers have “consistent, reliable and robust business processes to achieve quality objectives and promote continual improvement.” I delved into this in my first article. Some may ask “how does culture fit into QMM?”
Schein’s definition of organizational culture is often referred3 to as three levels:
- “artifacts that may reflect culture (for example, symbols and language),
- norms and values about appropriate attitudes and behaviours (espoused or real), and
- underlying assumptions and beliefs (conscious or unconscious).”
In short, the visible and the invisible.
If not everything is visible, it is perhaps difficult to consider what the impact of organizational culture might be on a quality system, its effectiveness and robustness, and even its maturity state. Furthermore, how would an organization measure the impact of these cultural attributes or go about improving culture to enhance quality maturity?
Why Getting It Right Is Important
It is apparent that organizations and regulators alike understand the very clear link between people within an organization and their behaviors and, indeed, the performance and compliance level of the organization. This in turn has a bearing on the organization’s success and the cost of quality, which is a factor in supply chain disruptions and therefore impacts patient outcomes.
Organizational culture change is not something that is developed overnight or implemented via a set of instructions, nor is it something that can be changed by minor adjustments to a few people’s behaviors. It takes time and effort. Most importantly, it takes sustained commitment, unwavering dedication to an aligned approach, robust and constructive minimization of distractions, and rapid management of activities out of alignment with the cultural transition.
The focus should be on a patient-centric organization taking collective accountability for quality, underpinned by inspirational leadership and an enhanced employee experience.
Strong Organizational Culture Has Clear Impact On Maturity Levels
We already know that organizations with lower levels of QMM tend to exhibit certain traits or characteristics, such as outdated procedures, procedures not being adequately or correctly followed, little to no proactive work on continuous improvement, minimal innovation, a low level of creativity, and higher than average levels of unplanned work resulting from deviations and excursions. Importantly, we have observed organizations in this maturity level to also have a fragmented culture (e.g., hierarchical fractures), lower levels of staff morale, and higher levels of attrition. Furthermore, this has, on occasion, resulted in a perceived or real risk of lower levels of fairness, inclusion, and/or equality. The link between low quality maturity and poor DEI staff survey results is a relatively new, but understandable, finding from NSF’s deployment of the QMM assessment. This finding marks a concerning state that can often be difficult to get out of.
Spotting The Doldrums
When a successful organization finds itself in a period of stagnation, it is important to first be able to recognize that there is a problem. As with all elements of quality maturity, leadership is critical in identifying this looming risk. Team leaders must actively scan conditions, the regulatory climate, and staff performance to look out for risks but also to avoid the doldrums — where the organization goes into a period of stagnation or listlessness. Leaders must look for early warning signals, track general company performance, assess the future landscape, observe competitors, and rally their team to identify unexpected changes in activity or performance.
To ensure that an organization does not get into the doldrums in the first place, organizational and quality leadership must identify the early warning signals or leading indicators. These signals must then be included in the metrics that are considered, monitored, and studied for shifting trends. Any indication of the organization going off course must be corrected with definitive actions that are well communicated inclusively, through teams.
A Five-Step Process To Kickstart Change
Unfortunately, even with active intervention, some organizations may still find themselves nearing, or indeed stuck in, a period of uneasy impassivity, with performance seemingly stalling. How, then, can an organization change course and make a pivot toward success?
There are several steps to take:
- Identify and recognize the current state.
- Analyze and assess the situation.
- Consider the root cause(s).
- Develop and implement a corrective and preventive plan.
- Monitor and check effectiveness.
This is a simple list and a process most will be familiar with, so why is it so difficult to do?
Getting an organization out of the doldrums is not just about correcting a process and procedure, although this may also be necessary. It is about changing culture and mindset — the way the organization works, looks, and feels. It is about alignment of the operations, policies, processes, and teams with the leader’s vision, instructions, and ambitions.
Recognizing that an organization is in the doldrums is perhaps the easy part. Getting it out takes sustained leadership commitment, coordinated effort across the organization, and long-term dedication from everyone to the agreed end goal.
Scope, Not Scale, Determines Analysis
A full analysis and assessment of the organization’s current state is critical to reaching the desired outcome. Without fully understanding where the challenges lie, it is difficult to rectify the situation. Equally, a partial analysis or segmented view could result in the organization following a different, and perhaps still advantageous course, but one that would be off course for full success.
How much analysis is enough?
This, of course, is an open-ended question. The answer is one of scope, not scale.
Third-party consultants are experienced practitioners and can more easily obtain a clear overview of an organization and its challenges by taking a broad look (scope). The level of depth (scale) required is dependent on several factors, such as size and complexity of the organization and if corrective action is required. It should be noted that the analysis is not a compliance review, audit, or inspection – although non-compliances or risks should not be ignored – rather, this is a holistic review of an organization; it is about how it operates, not what it does.
As a high-level framework, Galbraith’s Star Model4 is a useful starting point.
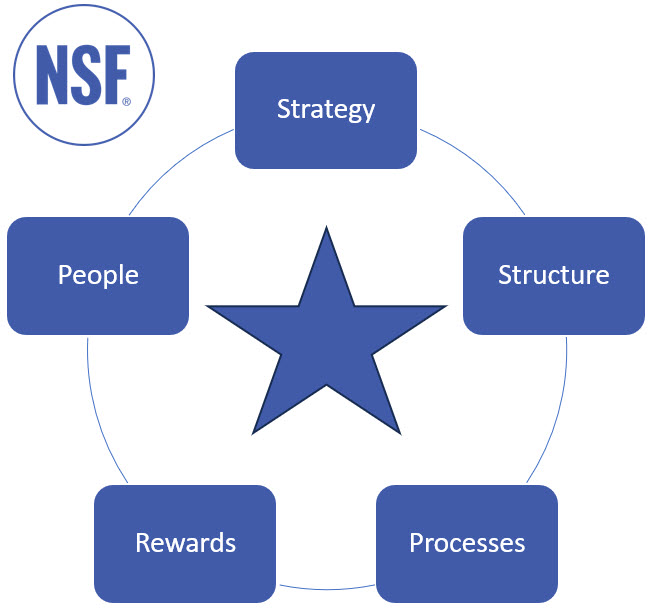
Figure 1: Galbraith’s Star Model4 (author’s adaptation of original)
- Strategy refers to direction. What are the corporate and strategic goals and how do they drive direction?
- Structure refers to organizational design, authority, and empowerment, but it ultimately identifies who has decision-making powers.
- Processes relate to information flows and how they are set up to enable your desired business model.
- Rewards drive motivation and incentivize employees in the direction of your desired business model, vision, and outcomes.
- People considers the skills, capabilities, and capacity requirements to support your desired business model.
The aim of the analysis and assessment is to look for alignment or, more importantly, misalignment.
First, however, the organization and its leadership must be clear on the direction, vision, and objectives for the future. There must be no doubt about the golden star the organization is following. This should flow like a thread throughout the organization — from the vision and mission to the organizational and quality strategies, and into team and personal objectives. Critically, it must be the guiding light that informs the analysis and assessment.
Tracking Toward Success Together
The journey begins with a plan of action to create alignment across the organization.
It is important to recognize that leadership — in its role taking collective accountability for the organization’s success — should take seriously any area that has difficulty in maintaining alignment with the desired business performance levels, culture objectives, or golden star. The leadership team as a collective should investigate, seek solutions, and work cohesively to resolve issues or deviations. Solutions should clearly demonstrate the entire leadership team supports the direction of travel.
Cohesion and unity are paramount when it comes to leadership action and communication.
It may be advantageous to identify role models to serve as an example of the behaviors and attributes desired from staff within an organization. Leadership roles naturally place individuals in this role model position. Role modelling provides a basis to set the culture and climate of an organization.
Role modelling requires those who undertake the role to demonstrate the culture and values of the organization. This is critical when going through a state of flux or change to re-engineer an organization back to full alignment. How leaders and role models behave impacts the employees’ perception of the organization. It can create a sense of empowerment by supporting the establishment of trusted relationships and it can enhance employee experience and engagement. It is well recognized that higher employee satisfaction leads to higher levels of performance.
QMM Serves As A Powerful Tool For Future Growth
Organizational culture can feel difficult to change, but it is the change from poor quality culture to rich quality culture that marks the organizations that are resilient, compliant, and able to prioritize patient outcomes.
From quality checklists to Galbraith’s Star Model, the tools identified in this article are just that: tools. The beating heart of QMM is people in the organization who are hungry for excellence. In the current economy, the financial benefits of growing quality maturity — savings due to decreased supply chain interruptions and employee turnover — are more important than ever. However, the intangible benefits, such as increased DEI goal attainability, lack of distractions, and ability to laser focus on patient outcomes, make QMM the most important goal an organization can set for 2024.
References
- March 2023. Fiscal Year 2022 Report on the State of Pharmaceutical Quality. (2023). Available at: https://www.fda.gov/media/169611/download [Accessed 5 Jul. 2023].
- Research, C. for D.E. and (2022). CDER Quality Management Maturity. FDA. [online] Available at https://www.fda.gov/drugs/pharmaceutical-quality-resources/cder-quality-management-maturity [Accessed 5 Jul. 2023].
- CIPD. (n.d.). CIPD | On this page. [online] Available at: https://www.cipd.org/en/knowledge/factsheets/organisation-culture-change-factsheet/. [Accessed 5 Jul. 2023].
- Galbraith, J. (2002). The star model. [online] Available at: https://www.jaygalbraith.com/images/pdfs/StarModel.pdf [Accessed 12 Dec. 2023].
About The Author:
 Samantha Atkinson, Ph.D., is executive vice president and principal consultant, NSF. She has 16 years of experience at the U.K. Medicines and Healthcare products Regulatory Agency (MHRA), where she had accountability for regulatory activities across the U.K. supply chain, including inspectorate, enforcement, licensing, clinical trials, pharmacopoeia, and premarket medical devices. Atkinson is an expert in organization or system transformation and reform, and at crisis management. She has overseen national and international incident management, and was the executive lead for the MHRA’s task force in response to the COVID-19 pandemic. Previously, she served as co-chair of the International Coalition of Medicines Regulatory Authorities (ICMRA) COVID-19 Working Group. Atkinson earned her BSc, MSc, and Ph.D. from University of Reading, UK; her MBA from University of Warwick, UK; and achieved the Major Projects Leadership Academy with the Saïd Business School at the University of Oxford, UK.
Samantha Atkinson, Ph.D., is executive vice president and principal consultant, NSF. She has 16 years of experience at the U.K. Medicines and Healthcare products Regulatory Agency (MHRA), where she had accountability for regulatory activities across the U.K. supply chain, including inspectorate, enforcement, licensing, clinical trials, pharmacopoeia, and premarket medical devices. Atkinson is an expert in organization or system transformation and reform, and at crisis management. She has overseen national and international incident management, and was the executive lead for the MHRA’s task force in response to the COVID-19 pandemic. Previously, she served as co-chair of the International Coalition of Medicines Regulatory Authorities (ICMRA) COVID-19 Working Group. Atkinson earned her BSc, MSc, and Ph.D. from University of Reading, UK; her MBA from University of Warwick, UK; and achieved the Major Projects Leadership Academy with the Saïd Business School at the University of Oxford, UK.
