Future-Ready Development

Ask us to strategize with you on your therapeutic development approach, design and execute experiments, plan, source, make and deliver your products, and help you write and review regulatory documents and interact with the agencies. We do more than a typical manufacturing organization, and we do it better.
Choose From Our One-Stop Solutions
Therapeutic Discovery Research
- CAR1 engineering and gene editing (viral/non-viral)
- Cellular product design and cell engineering
- Product quality profile development
- Candidate screening and selection
1 CAR: Chimeric antigen receptor
Process Development
- Cell therapies (autologous, allogeneic, iPSC1)
- Genome editing (viral/non-viral)
- Viral vector (AAV2, LVV3)
- mRNA
- Lipid nanoparticles (LNP)
- Extracellular vesicles (EV)
1 iPSC: Induced pluripotent stem cells
2 AAV: Adeno-associated virus
3 LVV: Lentiviral vector
Analytical Development
- Method development and optimization
- Flow cytometry
- PCR
- HPLC etc.
- Product characterization
GMP Manufacturing
- Viral vector (up to 200L scale)
- Cell processing
- mRNA enzymatic synthesis
- Lipid nanoparticle formulation
- Fill/Finish (vial and bag)
Quality Control
- Environmental monitoring
- In-process testing
- Specification testing
- Stability testing
- Method transfer, qualifications, and validation
Development CMC/Consulting
- Therapeutic discovery, development, CMC and regulatory strategies
- Writing and reviewing regulatory submissions
- Interactions with health authorities
State-Of-The-Art Facility
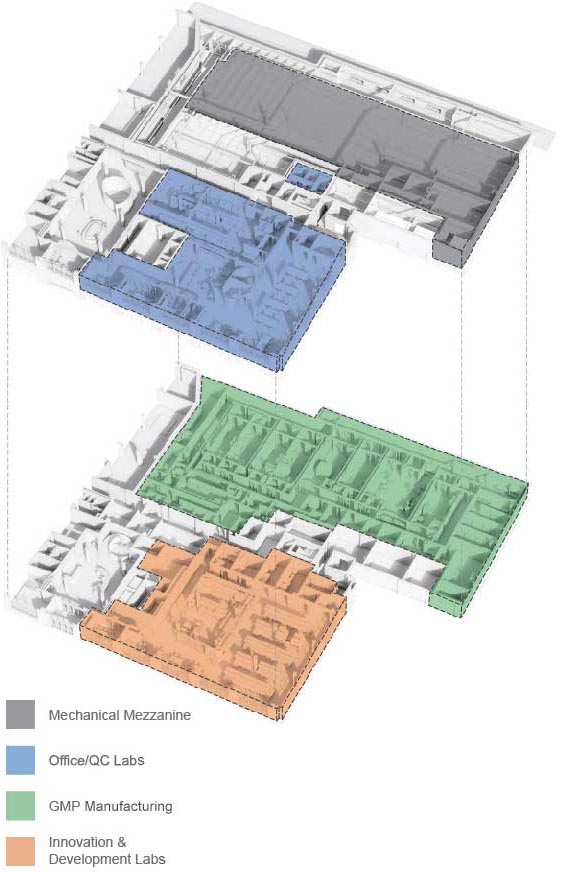
44,000 sq. ft. integrated facility in Watertown, MA
Innovation and Development Labs
- Process Development
- Analytical Development
- Translational Research
GMP Manufacturing
- 5 Cell Processing Suites
- 2 Viral Vector (upstream and downstream) Suites
- 1 mRNA and LNP Suite
- 1 Fill and Finish Suite
Quality Control Labs
Office Space
“Landmark Bio’s facility serves as a transformational development center for our talented research community to advance technologies that manufacture and distribute breakthrough therapies.”
— Cynthia Barnhart, MIT Provost, Landmark Bio Board Member
