Fermentation Of Live Biotherapeutic Products In cGMP Environments
By Jimi Kjærsgaard Pettersson, NIRAS

Live biotherapeutic products (LBP), which are mainly composed of anaerobic bacteria, are transforming the landscape of microbiome-centered medicine and advanced therapy products.
The next step in our educational series for Life Science Connect delves into the heart of LBP manufacturing: the fermentation process. (Editor’s note: Check out the first article in this series on drug facility design for live biotherapeutic products here.) In this article, we follow the journey from master cell bank to large-scale anaerobic fermentation, highlighting the unique regulatory and technical needs for working with oxygen-sensitive, strictly anaerobic therapeutic microbes in a cGMP setting.
Master Cell Bank: Foundation Of Manufacturing
The master cell bank (MCB) marks the beginning of production for strictly anaerobic bacterial strains, providing the quality foundation for all subsequent manufacturing steps. The MCB is established by selecting a single well-defined microbial strain or, in the case of multi-strain LBPs, a carefully composed combination of strains. Advanced genetic, phenotypic, and functional assays are used to ensure that each strain, or the entire consortium, meets all therapeutic and safety criteria. For anaerobic LBPs, special attention is given to the strain’s viability and stability under oxygen-free conditions.
Key Processes in MCB Production:
- Select and isolate the bacterial strains from an original source.
- Perform comprehensive screening for genetic identity, purity (free from contaminants), and desired characteristics.
- Expand under tightly regulated, anaerobic, and sterile culture conditions.
- Aliquot into sterile vials containing protective cryopreservation medium.
- Ensure controlled-rate freezing and storage in vapor-phase liquid nitrogen or ultra-low temperature freezers, minimizing oxygen exposure at all steps.
- Maintain extensive quality control (QC): sterility, viability, identity, and safety.
- Perform full cGMP documentation and traceability for regulatory compliance.
Why This Matters:
The reliability and reproducibility of every LBP batch, now and in the future, depend on the integrity of the MCB. Every process is validated and performed by highly trained personnel in cGMP-compliant facilities.
Working Cell Bank: Bridging Bench To Production
The working cell bank (WCB) is generated from the MCB and provides the immediate inoculum for production runs. This enables manufacturers to expand the LBP strain as needed while minimizing passages, thereby preserving genetic and phenotypic consistency.
Steps Involved:
- Thaw a vial from the MCB under anaerobic conditions.
- Subculture and expand the strain to create multiple aliquots.
- Perform QC at every stage to confirm identity and viability.
- Store the WCB under conditions validated for anaerobes (e.g., oxygen-free, low temperature).
Process Considerations:
- All manipulations, including thawing, expansion, and aliquoting, are performed either in anaerobic chambers or using specialized sealed bioprocessing bags and oxygen-scavenging agents.
- Documentation, environmental monitoring, and batch release are performed under strict cGMP.
Media Preparation: Ensuring An Anaerobic, Defined, And Protective Environment
Anaerobic growth media for LBPs must provide all nutrients required and be thoroughly deoxygenated to avoid oxidative stress. Media composition is carefully optimized to maximize cell growth while maintaining the desired traits of the therapeutic strain. A well-designed media does more than just support bacterial growth: it directly determines the robustness and stability of the microorganisms during fermentation. Media optimization typically occurs at the small scale during early development to support cell growth and enable initial assessment of therapeutic properties. While media composition is important for establishing metabolic activity and cellular integrity, successful performance during large-scale fermentation depends more critically on the control of fermentation parameters.
Scalability is not inherent; predictive models are employed to translate fermentation conditions to larger volumes, but direct scale-up outcomes are not guaranteed.
Importantly, this improved physiological resilience means that bacteria become less sensitive to oxygen in downstream process steps, reducing the risk of viability loss or damage if brief oxygen exposures occur during subsequent handling, formulation, or storage.
The redox potential of the media is always a critical factor for anaerobic bacterial strains, as maintaining the proper redox environment is essential for their optimal growth and resistance to oxidative stress. Redox potential can be fine-tuned with reducing agents (such as cysteine, sodium thioglycolate, or dithiothreitol) and is closely monitored to maintain a strongly reducing environment, especially for extremely oxygen-sensitive or obligate anaerobes.
Critical Steps:
- Use high-purity water and regulatory-grade raw materials, often subjected to additional filtration or pretreatment.
- Deoxygenate by sparging with, for example, nitrogen and, where needed, supplementing with reducing agents.
- Adjust and monitor the redox potential as required for strain-specific needs.
- Sterilize via autoclaving or filtration, followed by aseptic transfer to oxygen-impermeable vessels.
- QC completed media for sterility, pH, redox potential, and residual oxygen concentration.
Why It Matters:
The better the media is designed and controlled, the more resilient and high-quality the bacterial culture will be. This translates directly to improved yields, greater process robustness, and enhanced product stability throughout the entire manufacturing workflow, even in the face of inevitable minor process deviations or transient oxygen exposure.
Inoculation And Seed Culture: Preparing For Scale-Up
The stepwise transfer of the LBP strain from the WCB to the main fermenter is performed via a series of seed cultures:
- Thaw and perform initial expansion in small anaerobic vessels.
- Sequentially passage into larger seed bioreactors, with each step increasing cell mass while maintaining strict anaerobic conditions.
- Use closed, single-use vessels or glassware equipped with septa, airlocks, or pressure relief systems designed for oxygen exclusion.
- Check QC samples at each stage for purity, viability, and growth kinetics.
Anaerobic Expertise Required:
- Specialized transfer techniques, using syringe, pump, or peristaltic systems within anaerobic environments, can be adapted for different scales. For handling larger volumes, sterile oxygen-impermeable bags, designed specifically for anaerobic use, may be utilized. These bags allow for aseptic transfer and expansion of cultures while maintaining strict anaerobic conditions and can be integrated with closed system connectors to support high-volume seed cultures or process steps.
- Perform rigorous monitoring of environmental parameters (e.g., residual oxygen, redox potential).
- Take accurate cell density measurements using non-invasive optical sensors or rapid, closed sampling methods.
Anaerobic Fermentation: Scaling Up Under cGMP
The heart of LBP production is the main fermentation, where the cell mass for drug substance is generated.
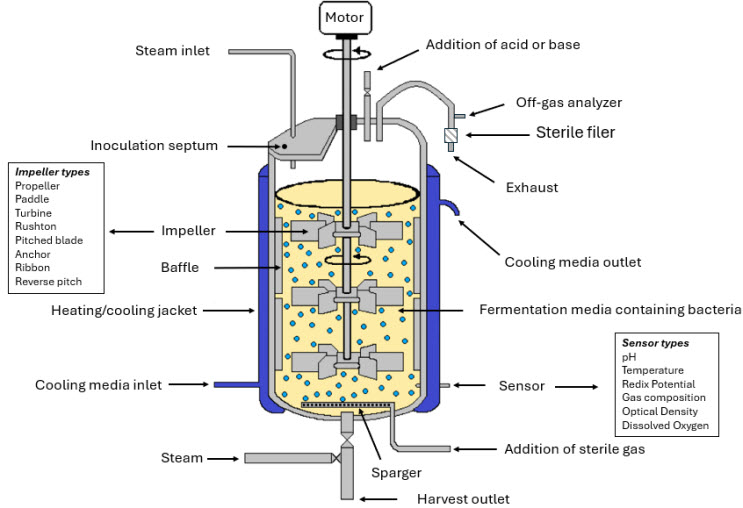
Figure 1: General illustration of an anaerobe fermenter.
The figure above provides a general illustration of an anaerobe fermenter and the illustration highlights key features such as closed, oxygen-impermeable vessel design, ports for sterile sampling and gas exchange, and integrated monitoring systems for parameters like temperature, pH, and redox potential.
The fermenter setup ensures strict exclusion of oxygen and supports aseptic handling throughout the fermentation process.
Fermenter Design Considerations
- Oxygen Control: Anaerobic fermenters are designed to prevent even trace oxygen ingress by using impermeable materials. The system is flushed with nitrogen or CO₂, and redox indicators provide continuous monitoring. Other oxygen-free gases or custom gas mixtures, such as argon or hydrogen blends, may also be used depending on process requirements and microbial tolerance, ensuring strict anaerobic conditions are maintained.
- Sterility: All connections are closed/sterile, and sampling is performed via dedicated anaerobic ports.
- Environmental Control: Continuous recording of pH, temperature, gas composition, and agitation speed occurs. Fermenters may be stainless steel or single-use bioreactors, all validated for anaerobic operation.
- Foam and Pressure Management: Because many anaerobic LBPs foam or generate gas, fermenters are equipped with controlled exhaust and antifoam addition systems.
- Impeller Type, Speed, and Shear Stress: The choice of impeller type and its operating speed significantly impacts mixing efficiency, mass transfer, and mechanical forces (shear stress) within the fermenter. Different impellers (such as Rushton turbines, pitched-blade, or marine propellers) generate varying flow patterns and shear environments that can either support healthy bacterial growth or cause damage to the anaerobic cells. It is therefore essential to select an impeller design that balances effective mixing with minimal shear stress to preserve microbial viability and productivity. Moreover, impeller speed must be carefully controlled during fermentation to optimize nutrient and metabolite distribution while avoiding excessive shear forces. High impeller speeds increase shear stress, which can negatively impact sensitive or filamentous anaerobic microbes, leading to lower yields or batch inconsistency. During scale-up and technology transfer, maintaining comparable impeller shear conditions is critical. Changes in impeller geometry, speed, or fermenter size can alter shear stress and mixing, affecting bacterial growth and product formation. Therefore, thorough process characterization and control of impeller parameters are vital to ensure consistent performance across production scales.
Monitoring the Fermentation
A comprehensive set of parameters is measured throughout fermentation to assess growth, metabolic performance, and process stability:
- Optical density (OD), typically at 600 nm, provides a rapid estimate of cell concentration by measuring light scattering. Samples are diluted if needed to remain in the linear range and may be correlated with viable cell counts.
- pH is continuously tracked and actively controlled to stay within optimal growth ranges, compensating for acid or base formation due to metabolism.
- Production of key metabolites, such as short-chain fatty acids, organic acids, or specific therapeutic compounds, is measured via rapid assays or chromatography to track product formation and inform harvest timing.
- Dissolved oxygen (DO) is kept extremely low in anaerobic processes; any rise can indicate leaks or process deviations that must be addressed immediately.
- Temperature is continuously monitored and regulated, as even slight deviations can affect growth rate, yield, or product quality.
- Redox potential, particularly important for strict anaerobes, ensures a sufficiently reducing environment; it is controlled via oxygen free gas blanketing and reducing agents in the medium.
- Substrate concentration, monitoring sugars, nitrogen sources, or other critical nutrients, ensures proper feeding strategy and prevents depletion or inhibitory excess.
- pCO₂ and headspace gas composition provides insight into metabolic activity and can be linked to growth stage or product formation.
- Foam formation is tracked in real time to ensure timely antifoam addition and avoid operational risks.
These combined measurements provide a detailed understanding of fermentation progress, enabling proactive adjustments for feed rates, gas flows, or harvest timing to maintain optimal anaerobic growth and product quality.
Operating the Fermentation
- Inoculate from the final seed culture vessel under strict anaerobic protocols.
- Control feeding of nutrients (batch or fed-batch mode), preventing substrate inhibition and maintaining redox balance.
- Perform ongoing process monitoring using the parameters above to guide adjustments and decision-making.
- Harvest through closed systems into oxygen-free containers, ensuring maximal viability and minimal oxidation.
Quality by Design
All process steps are validated and managed under cGMP. Documentation, audit trails, and continuous environmental monitoring guarantee compliance and product safety.
About The Author:
 Jimi Pettersson is expertise director at NIRAS, with over 15 years of experience driving R&D and innovation in life science and pharmaceutical development. He has led the advancement and implementation of novel technologies for live biotherapeutic products, specializing in fermentation, downstream processing, and advanced drying techniques. Pettersson has a strong track record in cross-functional leadership, strategic collaborations with academia and industry, and IP management. He holds an M.Sc. in dairy technology and an Executive MBA from DTU, combining scientific expertise with leadership in the life sciences sector.
Jimi Pettersson is expertise director at NIRAS, with over 15 years of experience driving R&D and innovation in life science and pharmaceutical development. He has led the advancement and implementation of novel technologies for live biotherapeutic products, specializing in fermentation, downstream processing, and advanced drying techniques. Pettersson has a strong track record in cross-functional leadership, strategic collaborations with academia and industry, and IP management. He holds an M.Sc. in dairy technology and an Executive MBA from DTU, combining scientific expertise with leadership in the life sciences sector.
