FDA Introduces Quality Management Maturity Program
By Peter H. Calcott, Ph.D., FRSC, president and CEO, Calcott Consulting LLC

We have seen improvements in pharmaceutical quality in the international scene driven by efforts by the International Conference for Harmonisation. Guidances such as Q8–Q121–5 are directed to improvements in development of products, quality systems, life cycle management, and quality risk management. These are all embraced by FDA, EMA, and the Japanese authorities, which are cosigners of these documents.
Meanwhile, a recent white paper by an FDA task force indicates that drug shortages are often driven by quality failures (62%).7,8 This task force indicated that improvements in quality would help alleviate these shortages. It also recognized that the market does not recognize or reward companies with mature quality systems.
The FDA program described in a new document, CDER’s Quality Management Maturity (QMM) Program: Practice Areas and Prototype Assessment Protocol Development,6 is a next logical step to drive better quality, more predictable outcomes, and supply chain assurance.
We are all familiar with CAPA (corrective action and preventive action), which drives improved corrections to prevent repeats of a problem and to prevent the problem where it has not happened yet in other similar systems. These are often labeled as reactive and proactive measures, respectively. The new program actually takes a further step into what I term “anticipatory” actions — that is, measures that are so proactive they are initiated even before we have an inkling of a problem. They go beyond the expectations of simple good manufacturing practices (GMP). Of course, everybody who understands continuous improvement knows that these measures improve quality, decrease waste, and set the stage for better, predictable production and improved economics. In other words, a superior business process results in not just improved business but also improved compliance.
Described in the new document, the FDA developed a questionnaire for manufacturers, which, when completed, must be validated by independent assessment in an open and transparent manner. This should be available from the FDA directly using contacts in the document, who will use private contractor experts. The teams used to assess the status could be FDA staff, FDA contractors, or a combination. Upon completion, you submit voluntarily for evaluation. Alternatively, perhaps you could use an independent resource such as a consultant to assess it. Whether you share with the FDA is your decision. The aim is for your survey answers to generate an action plan to move your organization to the new improved state. If your organization shares the survey answers with the FDA, the agency can then use them, in an anonymous manner, to demonstrate to industry how industry is doing and for companies to benchmark.
What exactly is the FDA proposing as the desired state of bio/pharmaceutical quality? The goal of the main body of the program is to create a state (and a way to assess it) that sets the stage for these anticipatory actions. It describes measures that will allow you to ensure you are way ahead of problems.
The FDA uses the term “quality management maturity (QMM).” But what exactly does it mean and entail? How will a company know when it has attained this quality management maturity? The FDA describes four measures that are part of the desired state:
- Quality culture: A state where quality is a major focus and driver, where the focus is on the patient and the way the products meet their needs.
- Advanced management practices: Practices that are anticipatory, not waiting for failure, and seeking out opportunity for improvement.
- Identification of where QM practices can be improved: Don’t wait for the QMS to fail but be continuously looking for ways to improve and remove risk before it strikes.
- Minimize risk to product availability: Be anticipatory in the supply chain and identify weaknesses in the supply chain and fix the problems before they surface.
What is really described here is continuous improvement taken to the extreme. The agency commissioned independent contractors to prepare a questionnaire that was used in two voluntary pilot programs supported by an advisory committee. The first pilot program was run with drug product manufacturers and the other included active pharmaceutical ingredient manufacturers. After the rollout, the advisory committee assessed the results and encouraged the FDA to roll out this program further.
Within the questionnaire are five separate sections describing the desired state and how to measure it. With this self-answered questionnaire, you can assess your current state so you would know how well you were doing.
1. Management Commitment To A Quality Culture
This is demonstrated by management that truly takes ownership of quality. They set expectations that are clearly communicated to the organization. Everybody within the organization understands the expectations and adjusts their work to reflect these expectations. This is ongoing; it is a journey and not a destination. It is not a pep meeting every year or a single email from the C-suite but, rather, it is constant engagement with the processes. It is exemplified by frequent in-depth reviews by management of not just technical performance but how well the pharmaceutical quality system (PQS) meets their business needs. These would often be an integral part of the executive committee meetings – not something that is covered only if time permits. This requires an effective communication channel to and from the CEO. With this engagement, everybody is focused on the commonly shared goals. ICH Q103 talks about this element.
2. Business Continuity
This requires expert knowledge of your supply chain. That is, you understand where your strengths are and, most importantly, where your weaknesses are. It requires anticipatory analysis of your processes, your sources, and your systems. Identifying weaknesses before they cause a failure allows you to get in and fix them well ahead of time. You never, or rarely, enter firefighting mode. It entails designing safeguards to prevent failure or at least early warning measures to signal weakness or potential failure that is imminent. A key measure here is how we react to the identification of issues. Robust, predictable mechanisms are central to success. Accomplishing this requires a detailed implementation of quality risk management (QRM), with all the associated elements, and life cycle management. ICH Q92 and ICH Q125 describe these processes in detail.
3. Advanced PQS
This is the requirement to have a compliance program and PQS that go beyond the check the box mentality or the questions “what does the regulatory authority want us to do?” or “where in the regulations does it say we should do this?”. In this state, your PQS is a superior business process that meets the business requirements while paying attention to the regulations. From my experience, my clients that focus on good processes while understanding the regulations have this advanced PQS. And they are in compliance. An integral part is an effective quality monitoring program of both our QMS as well as our technical processes. Development of key performance indicators (KPIs) helps to monitor our success: KPIs that drive the right behaviors are critical. In this desired state we are always ahead of the game and are never playing catch up (or at least very rarely). The use of QRM is a critical tool in these efforts (ICH Q9).2 ICH Q103 and ISO 90009 describe the desired state for which we are striving.
4. Technical Excellence
You have to understand your technical processes well to be able to predict potential weaknesses and strengths. We have moved from empirical process development to a state where we incorporate modern methods of process development into our programs. Terms such as quality by design, experimental design, and process capability are now commonplace in the industry. With this improved methodology, we know more about our processes. We know what is tried and true and we know where the risks and weaknesses are. With this knowledge, we can develop strategies to monitor or even control our processes so we can predict anomalies. ICH Q103 describes knowledge management, which plays a key role in making information available to all who need it to do their jobs. Gone are the days of “on a need-to-know basis” to prevent access to information. ICH Q8,1 Q11,4 and Q125 describe these desired states. Of course, the tools of QRM (ICH Q9)2 play heavily.
5. Employee Engagement And Empowerment
An engaged workforce that is empowered adds strength to the organization. You now have hundreds more eyes watching how things are going and catching problems before they are catastrophic. But how do you engage and empower employees? It actually circles back to point 1: management commitment to culture.
Figure 1 describes the relationship of management commitment to employee behavior. Management sets the tone for the culture that results in good policies and procedures (say what you do), good execution (do what I say), and good records of our work, decisions, and actions (prove it), all with a mindset of continuous improvement (improve it). This encourages employees to take ownership, understand their role in the big picture, and understand why they are doing what they are doing. For success here, you need a good feedback mechanism so employees know how they are contributing to success.
Figure 1 – Interplay Between Management and Workforce
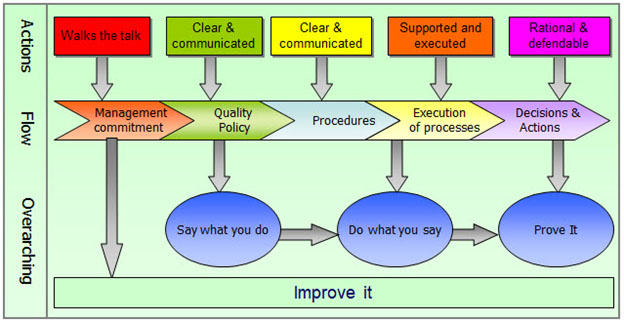
Of course, all five points are interlaced. Points 1 and 5 allow points 2, 3, and 4 to flourish.
The FDA also points out that this program or concept does not measure the GMP level and is neither a substitute for GMP nor for product review but rather tools that help the organization to assess its state. If done correctly, it is not an added burden but an integral part of doing business. In the long run, I agree, but in the short term it will take resources to set the program in motion. When implemented, the FDA hopes that these improved states can be used by companies as an added marketing tool to show customers the reliability of their processes and, hence, the superiority of their products.
The FDA points out that the principles here are not dependent on the size of an organization, the age of facilities, the number of products made, or where the company is in the development cycle. But these measures will help to improve or mature a quality culture and set a course for improved continuous improvement and end results.
References
- ICH Q8 – Pharmaceutical Development (2005)
- ICH Q9 – Quality Risk Management (2023)
- ICH Q10 – Pharmaceutical Quality systems (2008)
- ICH Q11 – Development and Manufacture of Drug Substance (2012)
- ICH Q12 –Technical and Regulatory Considerations for Pharmaceutical Lifecycle Management (2012)
- CDER’s Quality Management Maturity (QMM) Program: Practice Areas and Prototype Assessment Protocol Development (2023); https://www.fda.gov/media/171705/download?attachment
- https://www.outsourcedpharma.com/doc/fda-finds-drug-shortages-are-mostly-caused-by-quality-issues-0001
- Drug Shortages: Root causes and potential solutions: A Report by the Drug Shortages Task Force. June 2019, updated February 2020.
- ISO 9000 Quality Management Systems – Fundamentals and Vocabulary (2015)
About The Author:
 Peter H. Calcott, D.Phil., is president and CEO of Calcott Consulting LLC, which delivers solutions to pharmaceutical and biotechnology companies in the areas of corporate strategy, supply chain, quality, clinical development, regulatory affairs, corporate compliance, and enterprise e-solutions. He has also served as an expert witness. He also teaches at the University of California, Berkeley in the biotechnology and pharmaceutics postgraduate programs. Previously, he was executive VP at PDL BioPharma, chief quality officer at Chiron and Immunex Corporations, and director of quality assurance for SmithKline Beecham and for Bayer. He has also held positions in R&D, regulatory affairs, process development, and manufacturing at other major pharmaceutical companies. He has successfully licensed products in the biologics, drugs, and device sectors on all six continents. Calcott holds a doctorate in microbial physiology and biochemistry from the University of Sussex in England. He has been a consultant for more than 20 years to government, industry, and academia.
Peter H. Calcott, D.Phil., is president and CEO of Calcott Consulting LLC, which delivers solutions to pharmaceutical and biotechnology companies in the areas of corporate strategy, supply chain, quality, clinical development, regulatory affairs, corporate compliance, and enterprise e-solutions. He has also served as an expert witness. He also teaches at the University of California, Berkeley in the biotechnology and pharmaceutics postgraduate programs. Previously, he was executive VP at PDL BioPharma, chief quality officer at Chiron and Immunex Corporations, and director of quality assurance for SmithKline Beecham and for Bayer. He has also held positions in R&D, regulatory affairs, process development, and manufacturing at other major pharmaceutical companies. He has successfully licensed products in the biologics, drugs, and device sectors on all six continents. Calcott holds a doctorate in microbial physiology and biochemistry from the University of Sussex in England. He has been a consultant for more than 20 years to government, industry, and academia.
