FDA FY2019 Human Tissue And Cell Therapy Observations And Trends
By Barbara Unger, Unger Consulting Inc.
Very soon after publication of the FY2018 data on human tissue and cell therapy products, the FDA released the data from FY2019, which we address here. We examine data from FY2019 and a total of five years’ worth of trends in GMP inspection enforcement in this area. The regulations that govern this area, 21 CFR 1271, are the same regulations that govern segments of human cell therapy and gene therapy products. Human cell and gene therapy products are part of FDA’s Regenerative Medicine framework.
from FY2019, which we address here. We examine data from FY2019 and a total of five years’ worth of trends in GMP inspection enforcement in this area. The regulations that govern this area, 21 CFR 1271, are the same regulations that govern segments of human cell therapy and gene therapy products. Human cell and gene therapy products are part of FDA’s Regenerative Medicine framework.
The presentation of data herein differs from how it is presented on the FDA website, even though it uses the same raw data. For example, I combine all observation listings within a given section that cite 21 CFR 1271 into a single value. For example, §1271.47(a) and §1271.47(b) are consolidated into a single line item, §1271.47. Similar consolidation is done for each of the §1271 citations identified in Table 2. Consolidating the citations leads to slightly different conclusions. In my opinion, sometimes, slicing the observations so narrowly, as the FDA does, can dilute their impact. The FDA’s data includes only Form 483s issued through its electronic system. Thus, the data does not represent the FDA's complete collection of inspection observations for the year, and conclusions should be tempered by this limitation.
Many of the firms that are cited under §1271 are fertility clinics and the ever-increasing number of mostly unlicensed stem cell therapy firms. Many stem-cell firms do not produce minimally manipulated or homologous use cells, yet they continue to distribute them commercially and run afoul of the FDA, including several firms that received warning letters. Firms governed by §1271 that received warning letters in FY2019 include the University of Miami Reproductive and Fertility Center, Genetech Inc., StemGenex Biologic Laboratories LLC, Human Biologics of Texas/Globus Medical, Stratus Biosystems LLC, Stemell Inc., Family Fertility Center, Westlake IVF LLC, and Virginia Center for Reproductive Medicine. Most recently, in December 2019, FDA FY 2020, Liveyon Labs Inc. received a warning letter, for which the FDA published a press release explaining the warning letter.
The FDA published detailed guidance in November 2017 defining what constitutes minimally manipulated and homologous use in an effort to encourage stem cell firms to come into compliance and file the necessary INDs and BLAs for product approval. It also provides the FDA’s Compliance and Enforcement Policy, in section V.B., for this group of products. This year, Congress expressed frustration with the slow pace of firms coming into compliance with requirements and expectations. The FDA announced it is exercising “enforcement discretion” for three years after the governing guidance was published, and it is only taking action when the products pose a clear public health risk. Fortunately for the FDA, the courts upheld its authority in this area in a decision issued in June 2019. It seems that few of the firms are submitting INDs, even though this grace period will be over in late 2020, likely because too much money can be made in this area. It will be worth watching to see if there is a flurry of enforcement actions against these firms at the end of the enforcement discretion period, in November 2020.
Executive Summary
- Human tissue transplantation firms continue to receive only a fraction of the Form 483s issued to drug firms and, with the exception of FY2017, the number of 483s in this area has gradually increased. Particularly in FY2018, the number of Form 483s increased approximately 60 percent over FY2017 and the values continued to increase again in FY2019.
- FY2017 appears to be an anomaly when considering the five years covered in this analysis. The total number of Form 483s was substantially lower that year. It was, however, the only year of the five covered here where the number of observations regarding good tissue practices exceeded the number of observations for failure to meet donor eligibility requirements.
- Observations in the area of donor eligibility (§1271 Subpart C) clearly outpace the number of observations in the area of current good tissue practices (CGTP) (§1271 Subpart D) in all years except for FY2017.
- The number of observations addressing good tissue practices more than doubled in FY2019 from FY2018. The number of observations addressing donor eligibility requirements decreased from 2018 to 2019, though it remains higher than in the other years covered in Figure 1.
FDA Form 483 Inspection Observations
Figure 1 shows the number of Form 483s that have been issued in this product category over the last five fiscal years. Overall, eliminating FY2017, the number of 483s issued has gradually increased since FY2015 and I expect the number to continue to increase in FY2020.
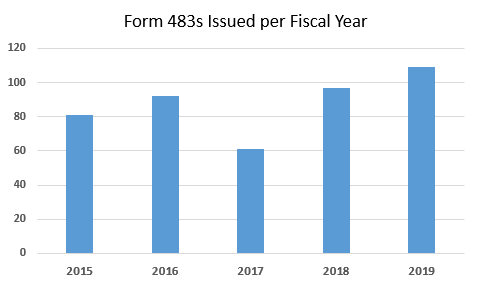
Figure 1: Total Form 483s issued FY2015 through FY2019
Table 1 presents the total number of observations for Subpart C and Subpart D from FY2015 through FY2019. Figure 2 shows the same data in a graphical presentation. For all years except for 2017, observations associated with donor eligibility requirements far exceeded observations associated with good tissue practices. In FY2019, as in FY2016, the number of good tissue practice observations approached the number of observations for donor eligibility requirements and has more than doubled from the values in FY2018.
Table 1: Total Number of Observations, FY2015 through FY2019
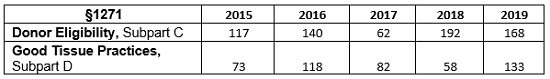
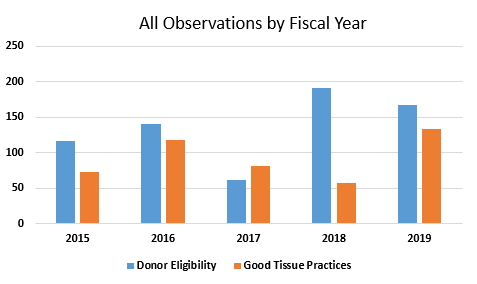
Figure 2: Total number of observations, FY2015 through FY2019
Table 2 shows the five most frequent inspection citations for donor eligibility requirements, Subpart C, and for good tissue practice requirements, Subpart D, between FY2015 and FY 2019. The areas that are new to the top five this year are highlighted in light gray. This year, §1271.85 is new to the top five and §1271.80 is no longer among the top five observations associated with donor eligibility. Changes to the top five in CGTP also appeared in FY2019. New to the group are §1271.200 and .270, replacing §1271.160 and .320. The relative numbers are small for most regulations cited in the observations, and I expect changes to happen from year to year in the top five for both groups.
Table 2: Human Tissue Product GMP Inspections, §1271 Citation Frequency per Fiscal Year
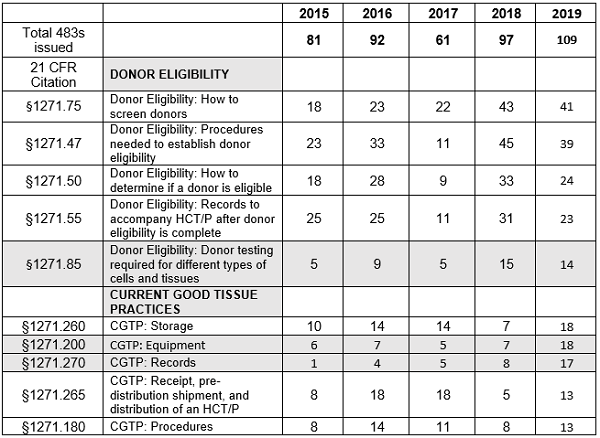
Figures 3 and 4 provide more granularity regarding the top five areas cited for both donor eligibility and CGTP based on values from FY2019. Figure 3 provides the five most frequently cited sections for observations addressing donor eligibility. In all sections cited, FY2019 values declined from those of FY2018, though for three of the areas, §1271.75, §1271.47, and §1271.85, the values in FY2019 remained significantly above those in other years.
Figure 4 shows marked increases in all five most frequent citations for CGTP for FY2019 when compared to FY2018. In two instances, §1271.265 and §1271.180, the values are lower than those in one or two of the previous years. It is noteworthy that observations associated with the requirement to establish and maintain a quality program are no longer in the top five in FY2019.
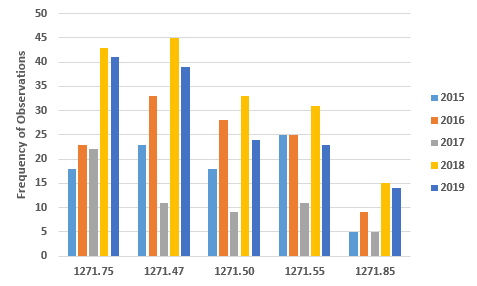
Figure 3: Top five observations from FY2019 associated with donor eligibility
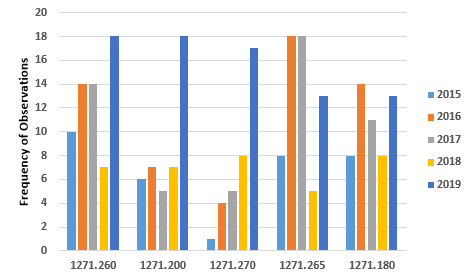
Figure 4: Top five observations from FY2019 associated with current good tissue practice
Conclusions
Human tissue transplantation includes important areas for current and future GMP inspections, reflecting the increase in the number of gene and cell therapy firms and stem cell clinics, including those that are not licensed. When enforcement discretion for stem cell clinics ceases in late 2020, we should expect a surge in inspections of these firms and subsequent enforcement actions, including warning letters and possibly seizures. This area can be complicated from a GMP perspective and includes the intersection of §1271 and §211, the GMPs for drug products, depending on the nature of the cell- or tissue-based product. The number of inspections, with the exception of FY2017, has increased over the past five years. The inspection focus for this group of products is primarily in the area of donor eligibility and the failure to document or perform adequate testing and medical record review of the donor. The potential infectious disease risks posed by human tissue or cell donations are the basis for this focus. CGTP observation frequency comes in a distant second to those in the area of donor eligibility. It will be useful to see if this trend continues in the coming years as more gene and cell therapies reach commercialization and stem cell clinics must conform to these requirements.
About The Author:
 Barbara Unger formed Unger Consulting, Inc. to provide GMP auditing and regulatory intelligence services to the pharmaceutical industry, including general GMP auditing and auditing and remediation in the area of data management and data integrity. Her auditing experience includes leadership of the Amgen corporate GMP audit group for APIs and quality systems. She also developed, implemented, and maintained the GMP regulatory intelligence program for eight years at Amgen. This included surveillance, analysis, and communication of GMP related legislation, regulations, guidance, and industry compliance enforcement trends. Unger was the first chairperson of the Rx-360 Monitoring and Reporting work group that summarized and published relevant GMP and supply chain related laws, regulations, and guidance. She is currently the co-lead of the Rx-360 Data Integrity Working Group. You can contact her at bwunger123@gmail.com.
Barbara Unger formed Unger Consulting, Inc. to provide GMP auditing and regulatory intelligence services to the pharmaceutical industry, including general GMP auditing and auditing and remediation in the area of data management and data integrity. Her auditing experience includes leadership of the Amgen corporate GMP audit group for APIs and quality systems. She also developed, implemented, and maintained the GMP regulatory intelligence program for eight years at Amgen. This included surveillance, analysis, and communication of GMP related legislation, regulations, guidance, and industry compliance enforcement trends. Unger was the first chairperson of the Rx-360 Monitoring and Reporting work group that summarized and published relevant GMP and supply chain related laws, regulations, and guidance. She is currently the co-lead of the Rx-360 Data Integrity Working Group. You can contact her at bwunger123@gmail.com.
