Empowering Patients With Self-Administration Drug Delivery Devices
Source: PCI Pharma Services
By Bill Welch, Executive Director of Market Development, PCI Pharma Services
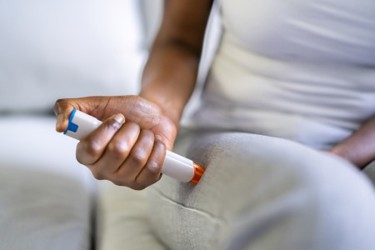
Integrating self-administered drug-device combination products into clinical trials benefits both patients and manufacturers. The market for self-administered injectable products is on the cusp of significant therapeutic advancements.
Beyond the usual industry buzzwords, the driving force behind this shift is necessity. This new era for drug-device combinations is addressing future healthcare needs. While it may prove to be revolutionary, it is, above all, a necessary and proactive development.
access the Article!
Log In
Get unlimited access to:
Trend and Thought Leadership Articles

Case Studies & White Papers

Extensive Product Database

Members-Only Premium Content

Welcome Back! Please Log In to Continue.
X
Enter your credentials below to log in. Not yet a member of Outsourced Pharma? Subscribe today.
Subscribe to Outsourced Pharma
X
Subscribe to Outsourced Pharma
