Emerging Trends In mAbs Manufacturing In 2025 And Beyond
By Bikash Chatterjee, CEO, Pharmatech Associates

The biopharma industry’s clinical pipeline is reaching its largest and most diverse in history. The number of distinct drugs in development has nearly doubled, growing from 3,200 in 2012 to 6,100 in 2022.1 Driven by the rising prevalence of chronic diseases like diabetes and rheumatoid arthritis and increased interest in GLP-1 antagonists, the monoclonal antibodies (mAbs) market is expanding at a compound annual growth rate (CAGR) of nearly 12% to exceed $236 billion by 2025.2 In the U.S. market, the FDA's recent adjustments to interchangeability requirements for biosimilars3 have streamlined the process of bringing a biosimilar to market.
The industry has continued to focus on process intensification as a means of shrinking processing times, reducing costs, and increasing productivity. Two key innovations — continuous perfusion in upstream processing and multicolumn chromatography in downstream processing — are having a profound impact on biologics manufacturing. These technologies promise to improve productivity, optimize resource utilization, and lower production expenses, making biologics more accessible to patients worldwide.
FDA Interchangeability Guidance
In a new draft biosimilar guidance for industry, the FDA is no longer recommending a repeated switching study to demonstrate interchangeability of biosimilars. Instead, the FDA will accept an assessment of why comparative analytical and clinical data in the application or supplement supports that the switching standard outlined in section 351(k)(4)(B) of the Public Health Service Act has been met. Applicants with a pending biologics license application (BLA) for a proposed biosimilar can submit an amendment with such an assessment for interchangeability review. This streamlines the biosimilar approval process.
This shift is expected to lower development costs and shorten timelines, encouraging more companies to enter the biosimilar market, with significant implications for physicians and their prescription practices. By eliminating the distinction between interchangeable and non-interchangeable biosimilars, physicians will have greater confidence in prescribing them, knowing that pharmacists can substitute them for the reference product at the pharmacy counter, similar to how generic drugs are handled. This ease of substitution is expected to enhance adoption rates among clinicians, improving patient access to lifesaving biologic therapies.
This increased accessibility and physician confidence are expected to fuel significant market growth for biosimilars. The relaxed guidance on interchangeability marks a crucial step in expanding the availability and affordability of life-changing biologic medications.
Fermentation: Higher And Higher Titers
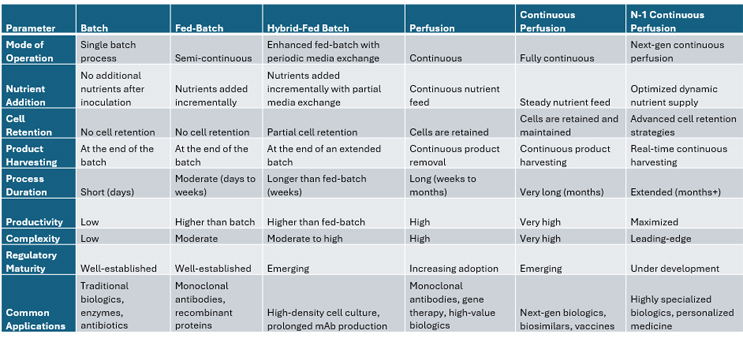
Through intensification and innovation processes, the effectiveness of cell culture fermentation has improved over the last few decades and, coupled with ever increasing productive cell lines, titer recoveries today have increased nearly tenfold. Initially, batch fermentation was the standard, where cells were cultured in a single batch stirred tank bioreactor until nutrients were depleted, leading to product harvest. This method, while straightforward, had limitations in productivity and consistency. To address these issues, fed-batch fermentation evolved, where nutrients are added incrementally during the culture process, extending the production phase and improving yields. However, in each of these approaches the accumulated waste or protein is not removed until the end of the culture period, which creates variability in the environment for protein recovery.
Perfusion fermentation further advanced this process step by continuously feeding fresh media into the bioreactor and removing spent media and products. This approach maintained optimal growth conditions, allowing for higher cell densities and consistent product quality. Hybrid fed-batch systems emerged as a compromise between fed-batch and perfusion to achieve higher cell densities and extended production cycles while keeping operational complexity lower than that of full continuous systems. Continuous perfusion took this a step further by maintaining a steady state of cell growth and product formation, enhancing efficiency and reducing downtime.
The latest innovation, N-1 continuous perfusion, involves using a perfusion system in the seed train stage (N-1) to produce a high-density cell culture that is then transferred to the production bioreactor. This method significantly reduces the time required to reach production scale, increases volumetric productivity, and improves overall process efficiency. These advancements have driven the biopharma industry to more efficient and scalable production methods.
Continuous Perfusion
Continuous perfusion offers significant advantages in biologics manufacturing, yet its adoption at a large scale has been gradual. However, momentum is building as the industry seeks more efficient, high-yield production methods. Several factors have contributed to the slower adoption, starting with the greater process complexity compared to fed-batch systems. Perfusion requires advanced control mechanisms to precisely regulate cell density, nutrient supply, and waste removal. Additionally, cell retention devices, such as tangential flow filters, must function continuously without clogging, adding to operational challenges.
Another key hurdle lies in CMC (chemistry, manufacturing, and controls) considerations. Demonstrating process stability and product consistency in a continuous system is inherently more complex than in traditional fed-batch processes, where discrete batches allow for well-defined quality control checkpoints. Lastly, not all cell lines thrive under perfusion conditions, necessitating further process development to maintain high yields and cell viability.
Despite these challenges, continuous perfusion is gaining traction, particularly for next-generation biologics such as gene and cell therapies, where efficiency, scalability, and cost-effectiveness are crucial. As downstream bottlenecks are addressed and regulatory frameworks evolve, continuous perfusion is poised to play an increasingly prominent role in the future of biologics manufacturing. A summary of each fermentation approach’s characteristics is shown below in Table 1.
Table 1: A Comparison of Fermentation Process Techniques. Click on image to enlarge.
Purification
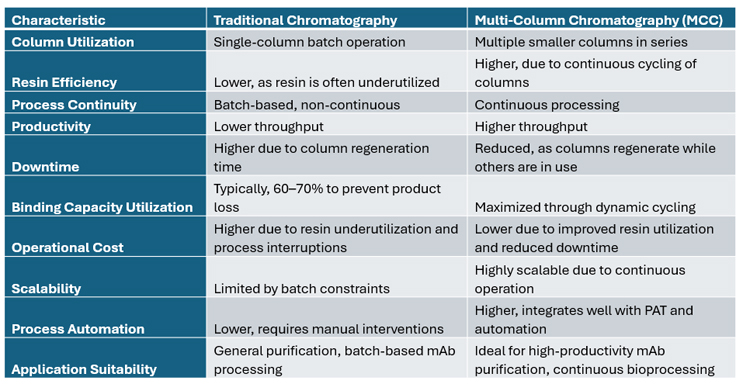
In biologics, we say, “the process is the product,” and downstream processing of high-titer recoveries comes with its own set of challenges. Multicolumn chromatography (MCC) is a cutting-edge downstream processing technique designed to enhance the efficiency and productivity of chromatographic separations. Unlike traditional batch chromatography, where a single column is used for sequential loading, washing, and elution, MCC employs multiple smaller columns arranged in series. This configuration enables continuous loading and processing, significantly improving resin utilization and minimizing downtime.
In MCC, product is initially loaded onto the first column, and any breakthrough is captured by subsequent columns. Once the first column reaches saturation, it undergoes washing, elution, and regeneration while the next column seamlessly takes over the loading phase. This cyclical process optimizes column usage, resulting in higher productivity, better resource efficiency, and reduced operational costs.
Compared to traditional chromatography methods, MCC offers distinct advantages. In conventional batch chromatography, resin capacity is often underutilized, with columns typically loaded to only 60%-70% of their dynamic binding capacity to avoid product loss. MCC, on the other hand, maximizes resin usage by continuously cycling columns through different phases, leading to more efficient material utilization while significantly reducing the need for large surge tanks and process interruptions.
MCC is particularly advantageous for the purification of mAbs, as it enables higher throughput, greater process consistency, and improved product quality. Recent innovations have further enhanced MCC's control strategies through the integration of machine learning and process analytical technology (PAT), paving the way for smarter, more adaptive bioprocessing solutions.
While MCC offers significant benefits for mAb purification, its adoption has been gradual for several reasons.
Unlike traditional batch chromatography, MCC involves synchronized operation of multiple smaller columns, requiring precise automation and real-time process monitoring. Any misalignment in column switching or flow rates can result in product loss or inconsistencies in purification efficiency. Another major hurdle is infrastructure and equipment requirements. Many existing batch-based facilities require significant retrofitting to integrate MCC, as it demands specialized hardware, real-time monitoring, and automated control systems.
Resin utilization presents another concern. While MCC improves resin efficiency, frequent cycling of columns can accelerate mechanical wear and degradation over time. Ensuring consistent resin performance requires careful monitoring and optimized replacement strategies to maintain binding capacity.
Lastly, MCC must be seamlessly integrated with upstream and downstream processes, particularly in facilities still operating in fed-batch mode. Continuous buffer preparation and real-time monitoring add additional operational complexity. Despite these challenges, advancements in automation, process control, and regulatory frameworks will drive greater adoption of MCC in the future of biologics manufacturing.
A summary of the key characteristics of traditional chromatography versus MCC is given below in Table 2.
Table 2: A Comparison of Traditional Chromatography and MCC Characteristics.
Click on image to enlarge.
Conclusion
The biopharma industry is undergoing a profound transformation, driven by the rising demand for biologics and the push for greater efficiency in manufacturing. The FDA’s recent guidance on biosimilar interchangeability represents a pivotal shift, lowering regulatory barriers, accelerating market entry, and fostering competition, which will enhance patient access to lifesaving therapies while driving affordability.
Biomanufacturers have long prioritized process intensification, and as demand continues to grow, they are increasingly adopting advanced, innovative upstream and downstream technologies. Continuous perfusion is transforming upstream processing, enabling higher productivity, improved process consistency, and enhanced scalability. Meanwhile, multicolumn chromatography is proving to be a powerful tool in downstream purification, allowing for efficient handling of high-titer recoveries while optimizing resource utilization and throughput.
Although adoption has been gradual, these innovations are gaining momentum, positioning the industry for greater efficiency, cost-effectiveness, and scalability in biologics manufacturing. These process optimization advancements are set to redefine the future of therapeutic production and patient accessibility worldwide.
The combination of regulatory flexibility, improved process efficiencies, and technological innovation will define the future of biologics manufacturing. Whether through N-1 continuous perfusion for faster production scaling or PAT-driven MCC for higher yields and purification efficiency, the industry is moving toward a more agile, cost-effective, and high-quality production landscape. The continued evolution of bioprocessing will improve drug availability and create a more sustainable and resilient global supply chain for critical biologic therapies.
References
- https://www.mckinsey.com/industries/life-sciences/our-insights/accelerating-clinical-trials-to-improve-biopharma-r-and-d-productivity
- https://www.thebusinessresearchcompany.com/report/monoclonal-antibodies-global-marketreport#:~:text=Monoclonal%20Antibodies%20(MAs)%20Market%20Size,(CAGR)%20of%2011.6%25
- Federal Register (2024, Jun 21). Considerations in Demonstrating Interchangeability With a Reference Product:
Federal Register: Considerations in Demonstrating Interchangeability With a Reference Product: Update; Draft Guidance for Industry; Availability [accessed July 4, 2024]
About The Author:
 Bikash Chatterjee, chief executive officer at Pharmatech Associates, has 30 years' experience in the design and development of pharmaceutical, biotech, medical device, and in vitro diagnostic products. His work has guided the approval and commercialization of over a dozen new products in the U.S. and Europe. Chatterjee is a member of the USP National Advisory Board and a past chairman of the Golden Gate Chapter of the American Society of Quality. He is the author of Applying Lean Six Sigma in the Pharmaceutical Industry (ISBN-13: 978-0566092046) and a keynote speaker at international conferences. Chatterjee holds a BA in biochemistry and a BS in chemical engineering from the University of California, San Diego.
Bikash Chatterjee, chief executive officer at Pharmatech Associates, has 30 years' experience in the design and development of pharmaceutical, biotech, medical device, and in vitro diagnostic products. His work has guided the approval and commercialization of over a dozen new products in the U.S. and Europe. Chatterjee is a member of the USP National Advisory Board and a past chairman of the Golden Gate Chapter of the American Society of Quality. He is the author of Applying Lean Six Sigma in the Pharmaceutical Industry (ISBN-13: 978-0566092046) and a keynote speaker at international conferences. Chatterjee holds a BA in biochemistry and a BS in chemical engineering from the University of California, San Diego.