Drug Characterization: A Key Factor In Autoinjector Design For More Challenging Formulations
Source: SMC Ltd.
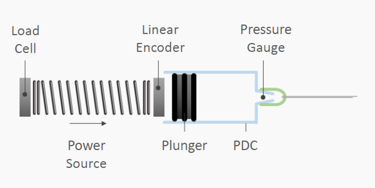
The most recent innovations in injectable formulations are beginning to push the limits of many traditional drug delivery platforms. Long acting injectables and biologics make up an increasing proportion of the drugs in development, with potential consequences including viscous formulations, complex fluid properties and high injection volumes. This article discusses the process used for the delivery of these formulations through developing an in depth understanding of their properties, and the unique challenges that they pose at the very earliest stage in the design process. Download the full paper for more information.
access the White Paper!
Log In
Get unlimited access to:
Trend and Thought Leadership Articles

Case Studies & White Papers

Extensive Product Database

Members-Only Premium Content

Welcome Back! Please Log In to Continue.
X
Enter your credentials below to log in. Not yet a member of Outsourced Pharma? Subscribe today.
Subscribe to Outsourced Pharma
X
Subscribe to Outsourced Pharma
