CRISPR And iPSC Disease Modeling And Drug Screening
By Jack (Jie) Huang, M.D., Ph.D.
In the study of human diseases, establishing reliable disease models has always been a key step in revealing the pathogenesis and developing therapies. However, traditional model systems, such as tumor cell lines and animal models, often have limitations such as large species differences, complex genetic backgrounds, and inability to accurately simulate human tissue pathological changes.1
In particular, in the study of neurodegenerative diseases, cardiomyopathy, and rare genetic diseases, the predictive ability of traditional models is insufficient.2 The development of induced pluripotent stem cell (iPSC) technology has provided a new path for in vitro modeling. iPSCs can be reprogrammed from patient somatic cells, retaining their complete genetic background information, and can be induced to differentiate into a variety of target cell types.3 When iPSC technology is combined with the CRISPR-Cas9 gene editing system, researchers can construct specific mutations or repair mutations in an isogenic background, thereby establishing highly accurate and controllable in vitro disease models.4 This platform has been widely used in research fields such as cardiovascular, neurological, metabolic, and hepatic, and renal diseases, and has shown unprecedented advantages in drug screening and etiology verification.5
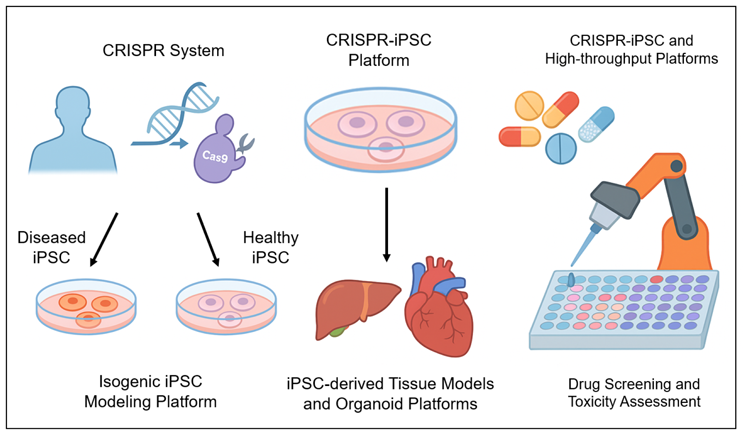
Fig 1. CRISPR and iPSC Disease Modeling and Drug Screening. This figure illustrates the CRISPR-iPSC system for disease modeling and drug screening. iPSCs derived from patients harboring disease-causing mutations can be corrected using the CRISPR-Cas9 system to treat single-gene diseases. The CRISPR-Cas9 system can also be used to generate iPSC-derived tissue models or organoid models (e.g., liver, heart). In addition, the CRISPR-Cas9 system can be combined with high-throughput screening technologies to evaluate the efficacy and toxicity of multiple compounds.
Isogenic iPSC Modeling Platform
Constructing isogenic disease models (isogenic iPSC pairs) is one of the key strategies for studying the function of gene mutations. This platform uses the CRISPR-Cas9 system to introduce or repair mutations at specific sites in iPSCs with the same genetic background, thereby obtaining cell lines with only a single genetic difference, greatly reducing the interference caused by background variation.6 This strategy not only improves the reliability of disease mechanism research, but also helps to improve the sensitivity and specificity of drug screening.7
The establishment of isogenic models usually includes two methods: first, introducing specific pathogenic mutations (knock-in) into iPSCs from healthy people to construct disease-mimicking cells; second, correcting the mutation site in patient iPSCs to obtain a "repaired" control.8 Common editing methods include Cas9 nuclease combined with ssODN donor template for homologous recombination (HDR), or using base editors to precisely repair single-base mutations .9 After editing is completed, stable mutant lines and control lines are screened through steps such as single-cell cloning, sequencing verification, and functional identification.
This platform has made progress in the study of various diseases. For example, in Alzheimer's disease research, isogenic neuron models established for APP and PSEN1 mutations have successfully reproduced early pathological changes such as Aβ deposition and tau phosphorylation10; in Parkinson's disease, iPSC neurons edited with the LRRK2 G2019S mutation exhibit mitochondrial dysfunction and dopaminergic neuron vulnerability11; amyotrophic lateral sclerosis (ALS) models are mostly based on SOD1, TARDBP, and C9orf72 mutations, revealing abnormalities in axonal conduction, RNA homeostasis, and stress granule formation in mutant motor neurons12; in addition, in the field of arrhythmias, cardiomyocytes with KCNQ1 or SCN5A mutations constructed using CRISPR have been widely used in drug risk assessment.13
In summary, isogenic CRISPR-iPSC models provide a powerful tool for accurate disease modeling and are increasingly becoming an important platform for human genetic disease research and precision medicine development.
iPSC-Derived Tissue Models And Organoid Platforms
Traditional two-dimensional cell culture models have significant limitations in simulating the human tissue microenvironment and multicellular interactions. In recent years, the use of iPSC-derived three-dimensional organoids to construct disease models that are closer to human tissue structure has become a research hotspot. When CRISPR gene editing technology is combined with the iPSC organoid platform, it is possible to accurately simulate developmental abnormalities or functional disorders caused by mutations at the organoid level, thereby improving the accuracy of disease research and drug screening.14
In terms of brain organoids, researchers have successfully introduced mutant genes associated with Alzheimer's disease, brain development abnormalities, autism, and other diseases, such as APP, MECP2, and CHD8, into iPSCs to construct mutant brain organoids for observing pathological features such as Aβ deposition, neural network development disorders, and axon guidance disorders.15 This type of three-dimensional nervous system model can better reflect the complex spatial differentiation pattern of human brain tissue and is suitable for studying the dynamic effects of mutations on brain development and cell type ratios.16 In liver organoid research, the CRISPR-iPSC platform has been used to simulate gene mutations such as ABCB11 and ATP7B that are associated with inherited liver diseases such as cholestasis and Wilson's disease.17
Mutant liver organoids exhibit specific functional defects in bile acid transport, copper metabolism, and drug detoxification, and can be used for mechanism research and targeted drug development.18 At the same time, these organoids are also widely used in drug metabolism kinetic analysis and hepatotoxicity assessment, improving the prospective and safety prediction capabilities of new drug screening.19
As a key model bridging the single cell and tissue levels, organoids provide an unprecedented tool platform for analyzing the mechanisms of complex human diseases and developing precise intervention methods. Their combination with CRISPR-iPSC is driving in vitro disease models from "structural simulation" to "functional reconstruction."
Drug Screening And Toxicity Assessment
Traditional drug development cycles are long and costly, and early animal models often lead to mispredicted efficacy or inaccurate toxicity assessments due to species differences.20 The integration of CRISPR and iPSC technology provides a new path for establishing personalized, precise, and high-throughput drug screening systems. In particular, isogenic mutation models constructed based on patient-derived iPSCs can truly reflect drug responses under specific genetic backgrounds, thereby accelerating the discovery and redevelopment of new drugs.
In high-throughput drug screening platforms (HTS), the CRISPR-iPSC system has several advantages: first, a large-scale cell model library can be established for different mutation backgrounds as screening targets; second, an automated differentiation platform can be used to quickly obtain uniform target cells, such as cardiomyocytes, neurons, and hepatocytes, to ensure screening consistency; third, mutation-corrected and mutation-carrying cells constitute isogenic controls, which can clearly distinguish specific drug efficacy from background interference.21
Researchers have successfully applied this system to targeted drug screening for rare genetic diseases.
For example, in a Parkinson's disease model associated with GBA mutations, after using CRISPR to construct mutant iPSC neurons, a class of small molecule drugs was screened through the HTS platform to promote the recovery of GCase enzyme activity and lysosomal function, and motor function improvement was subsequently observed in animal models.22
For another example, in lung organoids constructed from CFTR mutant iPSCs, researchers found a variety of small molecule candidates that have a corrective effect on the ΔF508 mutation through functional chloride channel detection, providing a basis for the classification and medication of cystic fibrosis patients.23
Another significant advantage is the assessment of individual differences in drug response. By constructing iPSCs from different patients and introducing the same mutation (or repairing the same mutation) separately, changes in drug sensitivity under different genetic backgrounds can be observed. This strategy has shown important value in drug screening for diseases such as long QT syndrome and amyotrophic lateral sclerosis (ALS) and helps to achieve "precision drug adaptation".24 The CRISPR-iPSC system also plays a key role in drug toxicity assessment. Applying drug candidates to iPSC-derived hepatocytes or cardiomyocytes allows for real-time monitoring of changes in cell function, toxicity accumulation, mitochondrial function, or cardiac electrical activity waveforms, which is superior to traditional animal models.25 In addition, by introducing mutations in specific metabolic-related gene polymorphisms (such as CYP2D6 and CYP3A5), the responses of people with different drug metabolism abilities can be simulated, thereby improving the accuracy of drug safety predictions .26
In summary, the CRISPR-iPSC integrated platform is gradually becoming an important support system for new drug discovery, repositioning, and personalized drug use. It not only improves screening efficiency, but also promotes the transformation of drug research and development toward precision, safety and efficiency.
Technical Challenges And Future Development
Although the CRISPR-iPSC system has shown great potential in disease modeling and drug development, its large-scale application still faces a series of technical and economic challenges.
First, off-target effects remain a core issue in gene editing safety, especially in long-term cultured iPSCs, where minor genomic changes may lead to phenotypic misjudgment or potential tumorigenicity.27 The use of high-fidelity Cas proteins and high-throughput sequencing screening strategies are currently the main means of circumvention.28
Second, model consistency and reproducibility still need to be improved. The differentiation efficiency of iPSC-derived cells is affected by multiple factors such as cell line differences and culture conditions, resulting in a lack of comparability of experimental results between different laboratories.29 In addition, the CRISPR editing success rate, clone screening and verification processes are time-consuming, further increasing the overall research cost.30
To address the above problems, artificial intelligence (AI) algorithms have been gradually introduced in recent years to predict efficient off-target sites, optimize gRNA design, assist in phenotypic data analysis and standardize modeling systems.31 At the same time, the organoid screening platform built on the automated microfluidic system has realized an integrated process of cell processing, differentiation induction, drug delivery, and phenotype collection, greatly improving experimental throughput and stability.32
In the future, if the CRISPR-iPSC platform can be further combined with AI and microengineering technology, it is expected to achieve comprehensive intelligence and industrialization of disease modeling and drug discovery.
Conclusion
The integration of CRISPR and iPSC technology has significantly improved the accuracy and functional reproducibility of human disease models, providing key support for the analysis of genetic mechanisms, targeted drug screening, and the development of personalized treatment strategies. This model platform is accelerating its transformation from basic research to clinical practice, becoming an important part of the precision medicine system.
References:
- Anna Loewa et al., Human disease models in drug development. Nature Reviews Bioengineering 2023, 1: 545-559. (https://doi.org/10.1038/s44222-023-00063-3)
- Jason Cousineau et al., Investigating the interplay between cardiovascular and neurodegenerative disease. Biology 2024, 13: 764. (doi: 10.3390/biology13100764)
- Jonas Cerneckis et al., Induced pluripotent stem cells (iPSCs): molecular mechanisms of induction and applications. Signal Transduction and Targeted Therapy 2024, 9: 112. (doi.org/10.1038/s41392-024-01809-0)
- Hyunsoo Jang et al., Chapter 10 - CRISPR/Cas9 technologies to manipulate human induced pluripotent stem cells. Methods in iPSC Technology 2021, 9: 249-287. (https://doi.org/10.1016/B978-0-323-85766-6.00012-7)
- Colin Walsh and Sha Jin, Induced pluripotent stem cells and CRISPR-Cas9 innovations for treating alpha-1 antitrypsin deficiency and glycogen storage diseases. Cells 2024, 13: 1052. (https://doi.org/10.3390/cells13121052)
- Martina Celotti et al., Protocol to create isogenic disease models from adult stem cell-derived organoids using next-generation CRISPR tools. STAR Protocols 2024, 5: 103189. (doi: 10.1016/j.xpro.2024.103189)
- Tuyana Malankhanova et al., A Human induced pluripotent stem cell-derived isogenic model of Huntington's disease based on neuronal cells has several relevant phenotypic abnormalities. Journal of Personalized Medicine 2020, 10: 215. (doi: 10.3390/jpm10040215)
- Metewo Enuameh et al., Developing isogenic cell models with CRISPR: an EML4-ALK fusion NSCLC cell line. Nature Portfolio 2020. (https://www.nature.com/articles/d42473-019-00011-z)
- Sachiko Okamoto et al., Highly efficient genome editing for single-base substitutions using optimized ssODNs with Cas9-RNPs. Scientific Reports 2019, 9: 4811. (https://doi.org/10.1038/s41598-019-41121-4)
- Dylan Kwart et al., A large panel of isogenic APP and PSEN1 mutant human iPSC neurons reveals shared endosomal abnormalities mediated by APP β-CTFs, not Aβ. Neuron 2019, 104: 256-270. (DOI: 10.1016/j.neuron.2019.07.010)
- Silas Buck et al., LRRK2-mediated mitochondrial dysfunction in Parkinson’s disease. Biochemical Journal 2025, 482: 721-739. (doi: 10.1042/BCJ20253062)
- Auderlan Gois et al., In vitro and in vivo models of amyotrophic lateral sclerosis: an updated overview. Brain Research Bulletin 2020, 159: 32-43.
- Eline Simons et al., iPSC-derived cardiomyocytes in inherited cardiac arrhythmias: pathomechanistic discovery and drug development. Biomedicines 2023, 11: 334. (doi: 10.3390/biomedicines11020334)
- Elisa Heinzelmann et al., iPSC-derived and patient-derived organoids: applications and challenges in scalability and reproducibility as pre-clinical models. Current Research in Toxicology 2024, 7: 1000197. (https://doi.org/10.1016/j.crtox.2024.100197)
- Marcella Birtele et al., Modelling human brain development and disease with organoids. Nature Reviews Molecular Cell Biology 2024, 26: 389-412. (https://doi.org/10.1038/s41580-024-00804-1)
- Hui Zhu et al., Recent advances in 3D models of the nervous system for neural regeneration research and drug development. Acta Biometerialia 2025, 202: 1-26. (https://doi.org/10.1016/j.actbio.2025.06.013)
- Romina Fiorotto et al., Liver diseases in the dish: iPSC and organoids as a new approach to modeling liver diseases. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 2019, 1865: 920-928. (https://doi.org/10.1016/j.bbadis.2018.08.038)
- Sen Liu et al., Liver organoids: updates on generation strategies and biomedical applications. Stem Cell Research & Therapy 2024, 15: 244. (doi: 10.1186/s13287-024-03865-3)
- Haoyu Fang et al., Human hepatobiliary organoids: recent advances in drug toxicity verification and drug screening. Biomolecules 2024, 14: 794. (doi: 10.3390/biom14070794)
- Thomas Hartung, The (misleading) role of animal models in drug development. Frontiers in Drug Discovery 2024, 4: 1355044. (https://doi.org/10.3389/fddsv.2024.1355044)
- Junyun Cheng et al., Massively parallel CRISPR-based genetic perturbation screening at single-cell resolution. Advanced Science 2022, 10: 2204484. (https://doi.org/10.1002/advs.202204484)
- Laura Smith and Anthony Schapira, GBA variants and Parkinson disease: mechanisms and treatments. Cells 2022, 11: 1261. (doi: 10.3390/cells11081261)
- Anna Demchenko et al., Human induced lung organoids: a promising tool for cystic fibrosis drug screening. International Journal of Molecular Sciences 2025, 26: 437. (DOI: 10.3390/ijms26020437)
- Volker Lauschke et al., Novel genetic and epigenetic factors of importance for inter-individual differences in drug disposition, response and toxicity. Pharmacology & Therapeutics 2019, 197: 122-152. (https://doi.org/10.1016/j.pharmthera.2019.01.002)
- Chun Liu et al., CRISPRi/a screens in human iPSC-cardiomyocytes identify glycolytic activation as a druggable target for doxorubicin-induced cardiotoxicity. Cell Stem Cell 2024, 31: 1760-1776. (DOI: 10.1016/j.stem.2024.10.007)
- Yitian Zhou and Volker Lauschke, The genetic landscape of major drug metabolizing cytochrome P450 genes—an updated analysis of population-scale sequencing data. The Pharmacogenomics Journal 2022, 22: 284-293. (https://doi.org/10.1038/s41397-022-00288-2)
- Tirthankar Sen and Rajkumar Thummer, CRISPR and iPSCs: Recent developments and future perspectives in neurodegenerative disease modelling, research, and therapeutics. Neurotoxicity Research 2022, 40: 1597-1623. (doi: 10.1007/s12640-022-00564-w)
- Magdalena Wojcik et al., High-throughput screening in protein engineering: recent advances and future perspectives. International Journal of Molecular Sciences 2015, 16: 24918-24945. (doi: 10.3390/ijms161024918)
- Viola Volpato and Caleb Webber, Addressing variability in iPSC-derived models of human disease: guidelines to promote reproducibility. Disease Models & Mechanisms 2020, 13: dmm042317. (doi: 10.1242/dmm.042317)
- Nasir Javaid and Sangdun Choi, CRISPR/Cas system and factors affecting its precision and efficiency. Frontiers in Cell Development Biology 2021, 9: 761709. (doi: 10.3389/fcell.2021.761709)
- Adib Rashid and MD Ashfakul Kausik, AI revolutionizing industries worldwide: A comprehensive overview of its diverse applications. Hybrid Advances 2024, 7: 100277. (https://doi.org/10.1016/j.hybadv.2024.100277)
- Brooke Schuster et al., Automated microfluidic platform for dynamic and combinatorial drug screening of tumor organoids. Nature Communications 2020, 11: 5271. (https://doi.org/10.1038/s41467-020-19058-4)
About The Author:
 Dr. Jack (Jie) Huang is currently the president at American Association for Scientist Entrepreneurship (AASE), the director at AASE Institute, the editor-in-chief of AASE Institute Journal (Science-to-Market Review), the vice president at American Botanical Drug Association (ABDA), the executive director of ABDA Journal (Botanical Drug), the visiting professor, chief scientist and founder/CEO at CSTEAM Biotechnology in Ohio. Dr. Huang is the author of chapters in two academic books, “Advanced Concepts and Strategies in Central Nervous System Tumors” and “Challenge of Glioblastoma - From Pathology to Survival,” and five medical science popularization books. His research and development interests have been focused on biological models, biochips, and CAR-T cell therapy.
Dr. Jack (Jie) Huang is currently the president at American Association for Scientist Entrepreneurship (AASE), the director at AASE Institute, the editor-in-chief of AASE Institute Journal (Science-to-Market Review), the vice president at American Botanical Drug Association (ABDA), the executive director of ABDA Journal (Botanical Drug), the visiting professor, chief scientist and founder/CEO at CSTEAM Biotechnology in Ohio. Dr. Huang is the author of chapters in two academic books, “Advanced Concepts and Strategies in Central Nervous System Tumors” and “Challenge of Glioblastoma - From Pathology to Survival,” and five medical science popularization books. His research and development interests have been focused on biological models, biochips, and CAR-T cell therapy.
