Contamination Control Strategies In Low Bioburden Biologic Drug Substance Manufacturing
By BioPhorum

The recently updated Annex 1 introduces the concept of a contamination control strategy (CCS). This is defined as: “A planned set of controls for microorganisms, endotoxin/pyrogen and particles, derived from current product and process understanding that assures process performance and product quality. The controls can include parameters and attributes related to active substance, excipient and drug product materials and components, facility and equipment operating conditions, in-process controls, finished product specifications, and the associated methods and frequency of monitoring and control.”
However, the term “control strategy” at many sites might be more narrowly defined as “parametric controls” for the wide range of critical quality attributes (CQAs) primarily focusing on unit operation critical process parameters, such as temperature and pH, which influence process performance indicators and/or CQAs. While CQAs may have included microbiological, particulate, or endotoxin factors, these may not have been historically considered as part of a holistic evaluation.
So how do manufacturers apply a CCS in a non-sterile world, particularly at a low bioburden biologic drug substance manufacturing site? How should the CCS address legacy facilities?
Much has already been written on the structure and creation of a CCS for sterile facilities (e.g., How to Develop and Document a Contamination Control Strategy from the ECA Task Force on Contamination Control Strategy) and non-sterile and low bioburden biologic drug substance (e.g., the Parenteral Drug Association’s TR90: Contamination Control Strategy Development).
Previously published documentation should be adapted for low bioburden drug substance manufacturing sites. However, in this article, we wanted to provide an interpretation of how the requirements of Annex 1 can be applied to give industry some enhanced clarity.
Quality Risk Management
For low bioburden biologic drug substance sites developing a CCS, it is important to follow quality risk management principles outlined in ICH Q9. Some elements of a sterile CCS may not be necessary for low bioburden sites. There may also be risks specific to a low bioburden biologic drug substance, e.g., sections on viral contamination risk factors for mammalian cell culture-derived products.
The CCS process should identify potential risks, assess potential controls and mitigations, and outline how the site will test, control, and monitor the effectiveness of these controls and mitigations. The CCS should differentiate between controls and monitoring. The acceptance criteria may be less rigorous than required for product sterility, but the core goal of minimizing contamination is the same in both sterile and non-sterile areas. For this reason, subject matter experts should consider the degree of formality in their risk analysis. More rigorous quality risk management tools should be applied where there is greater risk or complexity, and less intensive tools may be applied where there is less risk or complexity. Low bioburden biologic drug substance manufacturing can also use the intrinsic properties of the process conditions, where applicable, to minimize contamination risks.
Contamination Control Strategy Development Process
A CCS is a five-step process with ongoing risk assessment at its heart. The initial risk assessment for the area, facility (site), or process should inform the CCS, with periodic reviews and updates as required.
- Identify potential types of contamination and their sources. This is based on the types of products manufactured at the site, the processes involved in manufacturing, packing, storage, handling, equipment, personnel, and materials used. This should be done by conducting a thorough risk assessment of the manufacturing process with cross-functional subject matter experts (e.g., engineering, technical, quality).
- Outline the control measures in place (or to be put in place for a new build or modification) to prevent contamination. This may include implementing cleaning procedures for equipment, using dedicated areas for specific processes, and establishing procedures for personnel hygiene and gowning.
- Outline the applicable detection measures. Include clear links to instructions (procedures) for monitoring to ensure that contamination is detected and addressed promptly. This should include routine testing and testing in response to specific incidents or concerns.
- Document the CCS clearly and link it to the risk assessments and standard operating procedures that affect the controls summarized. Maintain records of monitoring and other relevant data and ensure these are easily accessible for review.
- Ensure the strategy remains practical, effective, and up to date with regular reviews and updates. Any changes to the manufacturing process, support processes (e.g., cleaning, heating, ventilation and air-conditioning systems, clean utilities, etc.), equipment, or materials should be evaluated for potential impact on the CCS, and the strategy updated accordingly. A defined process for performing this evaluation is important. This should be performed for each change (including post-implementation checks if required) and a regular review should be performed at a specified frequency to determine the holistic impact of accumulated changes over time.
Document Structure
A CCS needs to be documented and combined into a single comprehensive document. It can be written as a summary document, with links to the associated more detailed site procedures and policies that describe the multifaceted aspects of the control.
It is important for low bioburden biologic drug substance manufacturing sites to evaluate the scope of the CCS. Sites with multiple facilities (buildings), lines, or products should consider whether it makes sense to craft multiple CCSs or a single more complex CCS with associated challenges, including change management and increased content. Implementing a site master CCS with area- or product-specific CCS could be helpful. This approach allows for common areas across the site to be addressed at the site level. Meanwhile, the area- or product-specific CCS would focus on controls at a unit operational or product level, covering aspects such as design, implementation, and ongoing control of contamination specific to the area or product.
From Annex 1, elements to be considered within a CCS should include areas such as:
- types of product processes and principles of operation
- design of the plant and processes, including the associated documentation
- premises and equipment
- personnel
- utilities (water, pure steam, gases).
However, these should also align with the scope of Annex 1 and clearly reference the sections that the site has chosen to apply and comply with.
USP <1115> Bioburden Control of Nonsterile Drug Substances and Products also provides a useful graphic of factors contributing to non-sterile product bioburden (see Figure 1). The document also has a section on microbial control of drug substance manufacturing.
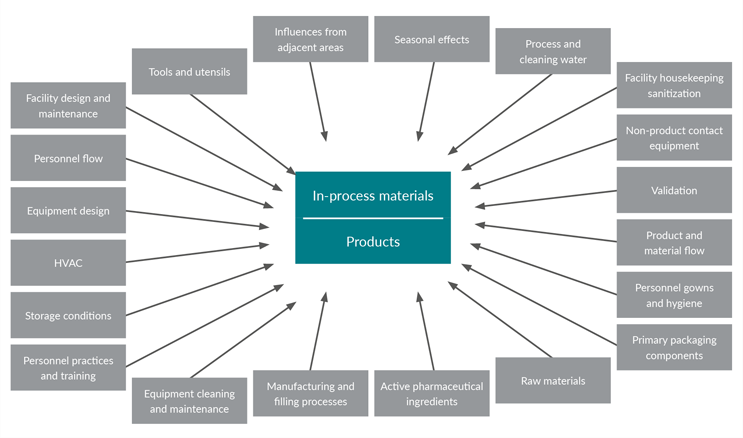
Figure 1: Factors that contribute to non-sterile product bioburden from USP <1115>
Document Controls
The CCS will likely fall within a company’s standard document control systems and be managed through the regular review, approval, and update processes. It is important to consider aspects such as referencing other documents by name rather than by version number to minimize the need for frequent updates.
Revisions to the CCS are expected, especially in the early years of implementation. The effectiveness of contamination control should be assessed for all changes, including changes prompted by CAPA (corrective and preventive actions) from change controls and/or deviations. The CCS should be reviewed at a frequency deemed appropriate by the site to ensure continued effectiveness and alignment with current practices. CCS topics should also be incorporated into an (annual) product quality review.
A well-maintained CCS provides a clear framework for implementing and monitoring contamination control measures, facilitating communication among stakeholders, and supporting continuous improvement initiatives.
Quality Implications
The CCS aims to enhance patient safety by improving product quality by minimizing contamination risks. Proactive quality risk management will identify, assess, and control contamination risks, prioritizing prevention through well-designed facilities, equipment, processes, and procedures. The CCS should not just be seen as a regulatory requirement but as a tool to promote understanding of contamination control at the highest levels of leadership, fostering a culture of continuous improvement. This can be done by ensuring the CCS is reviewed at the highest levels of site leadership.
Legacy Sites
While the principles of generating a CCS remain consistent, legacy sites present unique challenges that require careful consideration and adaptation of the approach outlined above. Areas to consider include:
- Acknowledge the gaps and the risks – Legacy facilities, often designed and built before the latest Annex 1 requirements, are more likely to have gaps in their CCS. Recognizing and documenting these gaps is crucial to enable prioritization of actions based on risk, thereby focusing on meaningful improvements.
- Leverage existing systems and data – While legacy sites might lack the most up-to-date systems, equipment, or design, they often possess valuable historical data that can be leveraged to inform the CCS. The review of existing documentation, risk assessments, monitoring data, and deviation records can provide insights into contamination trends, historical issues, and the effectiveness of current control measures.
- Prioritize risk-based decision-making – Risk assessment is particularly critical in legacy sites, where a phased approach might be necessary. Focusing on high-risk areas to ensure the most critical gaps are addressed first allows for more manageable, cost-effective improvements.
Conclusion
Annex 1 emphasizes the importance of a risk-based and holistic approach to contamination control. By carefully considering these factors, a robust and effective CCS can be developed, one that not only enhances product quality but also ensures patient safety.
This article summarizes the main points from a recent BioPhorum publication on this topic, which provides more details on the five-step CCS process. To learn more, check out the full paper, Contamination control strategies (CCS) in low bioburden biologic drug substance manufacturing: insights from BioPhorum.
