Considerations For Migrating A Drug Product Presentation From Vial To Pre-Filled Syringe
By Martin Gonzalez, Ph.D., Sr. Manager Formulation and Process Development, Pfizer CentreOne
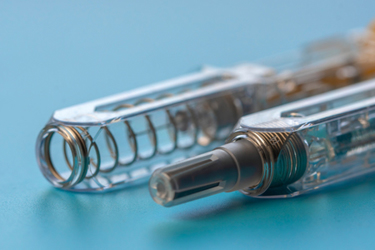
There is an upward trend for pharmaceutical companies to ask their contract development and manufacturing organizations (CDMO) to support the conversion of an existing product presentation into a different final container type. More specifically, providing options for moving a drug product from a vial into a pre-filled syringe (PFS).
There are many reasons why a drug developer may be interested in converting a drug product currently provided in a vial form into a PFS. It is sometimes driven by quality requirements to preserve product safety or potency. However, more often than not, the desire to create more patient-centric administration formats and to extend patents are underpinning the move.
In this article, Martin Gonzalez, Ph.D., Sr. Manager Formulation and Process Development at Pfizer CentreOne, discusses some of the most relevant engineering activities that are necessary to successfully transfer a product from vial into a PFS presentation.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Outsourced Pharma? Subscribe today.
