Case Studies: Is AI Making Biologics And CGT Outsourcing Smarter?
By Vadim Klyushnichenko, Outsourced Pharma Board Member

This continues our discussion of AI and CDMOs, focusing on biologics and cell and gene therapy (CGT) relationships. Part one is here.
AI Dashboards for Real-Time Process Monitoring
Cell therapy product development is inherently complex, involving multiple specialized steps often distributed across a global network of Contract Development and Manufacturing Organizations (CDMOs). As illustrated in the case of a first-generation switchable CAR-T cell therapy program developed at Calibr, five different CDMOs were engaged to execute key functions ranging from plasmid and lentiviral vector production to CAR-T cell manufacturing, Fab-switch drug substance development, and final fill-and-finish.
Each stage introduced unique operational and quality control challenges, requiring real-time monitoring of the supply chain, batch status, and technology transfers.1,2 Additionally, a dedicated cold chain logistics system had to be developed to ensure the safe and timely distribution of the CAR-T cell product to clinical trial sites, underscoring the need for continuous oversight and coordination across facilities.3
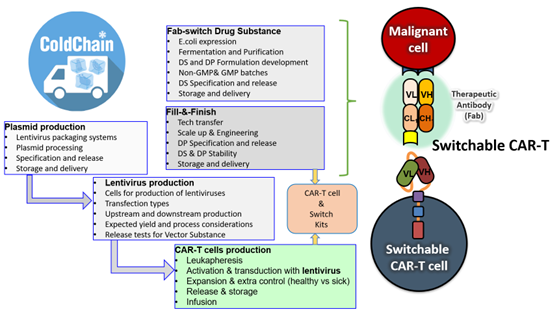
Figure 2: Distributed Manufacturing Workflow for Switchable CAR-T Cell Therapy Across Five CDMOs
Modern AI-powered dashboards can aggregate and analyze production data across multiple runs, sites, and equipment lines, offering intelligent summaries and alerts based on pre-defined and learned performance patterns.
Key applications include:
- Batch consistency tracking: AI models continuously evaluate critical quality attributes (CQAs) such as protein titer, cell viability, impurity levels, and product yield, flagging outliers and trend shifts that might otherwise go unnoticed until release testing.
- Microbial contamination surveillance: By integrating sensor data (e.g., temperature, humidity, airflow), and environmental monitoring results, AI systems can detect early warning signs of potential contamination, allowing for swift intervention before product loss or facility impact.
These dashboards serve as centralized command centers, enabling both sponsors and CDMOs to view and respond to process data in real time.
Predictive AI for Deviation and Failure Forecasting
Beyond monitoring, AI models forecast potential failures by analyzing historical and in-process data. Examples include:
- Cell growth curve anomalies: Early warnings on poor expansion or viability
- Vector batch failures: Pattern recognition across transfection and downstream metrics to prevent process loss
These predictive tools are especially critical in CGT, where each batch is high-value, low-volume, and often patient-specific. AI dashboards enable sponsors to shift from reactive troubleshooting to proactive quality control, ensuring better outcomes in multi-site, multi-step therapeutic manufacturing.
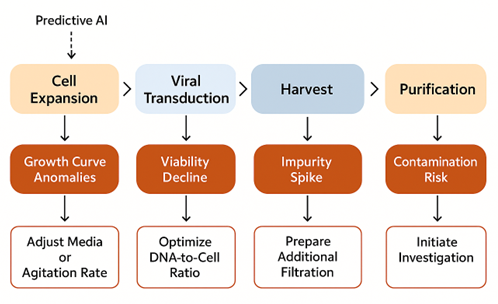
Figure 3. The Illustration on how predictive AI triggers and interventions across the manufacturing cycle
Case Studies: Applications of AI in CDMO Oversight
As AI integration in biopharmaceutical manufacturing matures, its value is increasingly evident across a range of therapeutic modalities and operational use cases. Below are two case studies that illustrative scenarios based on real industry trends, AI vendor capabilities, and common challenges observed in biopharma manufacturing. They represent emerging patterns seen across regulatory filings, quality audits, and supply chain case reviews.4-6
Case Study 1: Biologics – AI-Enabled Coordination Across Multiple CDMOs for ADC Manufacturing
A clinical-stage oncology company was advancing a novel antibody-drug conjugate (ADC) targeting a solid tumor antigen. Like many ADC programs, development and manufacturing required specialized capabilities that no single CDMO could fully provide. The workflow was split across four CDMOs, each responsible for distinct stages:
- CDMO 1: Monoclonal antibody production under GMP
- CDMO 2: Cytotoxic drug-linker synthesis under high-containment conditions
- CDMO 3: Conjugation and purification in an isolated bioconjugation suite
- CDMO 4: Fill-finish and labeling of the final drug product
This fragmented model created high operational risk due to cross-CDMO interdependencies, lead time mismatches, and differing quality system maturity levels. To manage this, the sponsor implemented an AI-powered supply chain and operational intelligence platform that:
- Mapped timelines, document transfers, and batch hand-offs across the entire CDMO network using real-time project data.
- Used predictive analytics to forecast delays in raw material arrival, release testing bottlenecks, and QA document turnaround.
- Integrated site-specific risk factors such as historical audit findings, equipment maintenance logs, and workforce turnover to dynamically assess risk exposure at each node.
- Provided automated alerts when slippage at one CDMO threatened downstream deliverables, enabling preemptive scheduling changes or expedited shipments.
The AI platform helped the sponsor align production windows, mitigate critical path delays, and ensure on-time release and delivery of the final ADC drug product to clinical sites. By synchronizing operations across multiple vendors, the company achieved a consistent clinical supply rhythm, maintained GMP compliance across interfaces, and reduced cumulative manufacturing cycle time by over 20%.
Case Study 2: Cell & Gene Therapy – Cold Chain Risk Mapping with AI
A CGT sponsor faced repeated losses in viral vector potency during clinical shipments. Investigations revealed temperature excursions and dwell-time mismatches across multiple transit hubs, but root causes remained elusive.
The company implemented an AI-enabled cold chain analytics platform that:
- Modeled historical shipping data across lanes, carriers, and seasonal variables.
- Integrated real-time environmental sensor data from shippers to identify recurring points of failure.
- Predicted probability of vector degradation based on temperature duration curves, humidity, and transit delays.
The tool flagged two hubs with high excursion risks and recommended rerouting via alternative logistics partners. Over the following quarter, the rate of compromised shipments fell by >80%, preserving product integrity and accelerating site activation timelines.
Future Outlook: Digital Twins And Intelligent Oversight
As therapies become more personalized and complex, AI is reshaping how sponsors manage CDMO relationships, especially when combined with digital twins and blockchain. A digital twin is a virtual replica of a manufacturing process, continuously updated with real-time data. In CGT, where timelines are tight and variability is high, digital twins enable simulation and optimization of production without disrupting live operations.
When integrated with AI, they can predict key quality metrics like cell viability or sterility, simulate batch behavior, and support proactive process control. Platforms like those from Intelligen, Inc. help model yield, raw material usage, and bottlenecks across upstream and downstream steps.7
Blockchain further enhances oversight by enabling secure, end-to-end traceability—critical for autologous therapies. When paired with AI, it ensures real-time patient-product matching and flags documentation or logistics risks.
Regulators are increasingly receptive to these innovations. Initiatives like the FDA’s Emerging Technology Program and EMA’s Innovation Task Force signal growing support for AI-based manufacturing tools, so long as they are explainable, validated, and compliant with data integrity standards.8,9
Conclusion
Artificial intelligence is redefining CDMO selection and oversight, from data parsing and predictive risk scoring to robotic execution and digital twin simulation. As biologics and CGT programs become more global and complex, manual approaches fall short. Sponsors that invest in AI-driven infrastructure today will gain speed, control, and resilience, positioning themselves to lead in the next era of intelligent therapeutic manufacturing.
References
- Nikolenko L., et al., First in Human Study of an on/Off Switchable CAR-T Cell Platform Targeting CD19 for B Cell Malignancies (CLBR001 + SWI019). Blood (2021), V.138 (Supplement 1): 2822. https://doi.org/10.1182/blood-2021-151727
- Klyushnichenko, Development of modern biologics and cell therapy products through global CMOs, BioProcess International West, San Diego March 21, 2025
- Klyushnichenko. Designing a Cold Chain Now to Support what your Products will be in the Future, American Biomanufacturing Summit, San Diego, May 2017.
- Jackson et.al., Revolutionize cold chain: an AI/ML driven approach to overcome capacity shortages. INTERNATIONAL JOURNAL OF PRODUCTION RESEARCH 2025, VOL. 63, NO. 6, 2190–2212. https://doi.org/10.1080/00207543.2024.2398583
- Tim Hafke, AI in Biopharma: Use Cases and Considerations, August 19, 2024, https://www.alpha-sense.com/blog/trends/ai-in-biopharma/?utm_source=chatgpt.com
- Anat Cohen, AI-Powered Dashboards: Transforming Real-Time Decisions in Pharma https://www.allex.ai/blog/ai-powered-dashboards-transforming-real-time-decisions-in-pharma?utm_source=chatgpt.com
- Da Gama Ferreira R., et al., (2023) Cell and Gene Therapy Manufacturing – Process Modeling and Techno-Economic Assessment (TEA) using SuperPro Designer. Research Gate DOI: 10.13140/RG.2.2.16363.00809
- Emerging Technology Program (ETP) https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/emerging-technology-program-etp
- Innovation Task Force (ITF) https://www.ema.europa.eu/en/human-regulatory-overview/research-development/supporting-innovation
---------
 Vadim Klyushnichenko, Ph.D., is VP Pharmaceutical Development & Quality, California Institute for Biomedical Research (Calibr)
Vadim Klyushnichenko, Ph.D., is VP Pharmaceutical Development & Quality, California Institute for Biomedical Research (Calibr)
